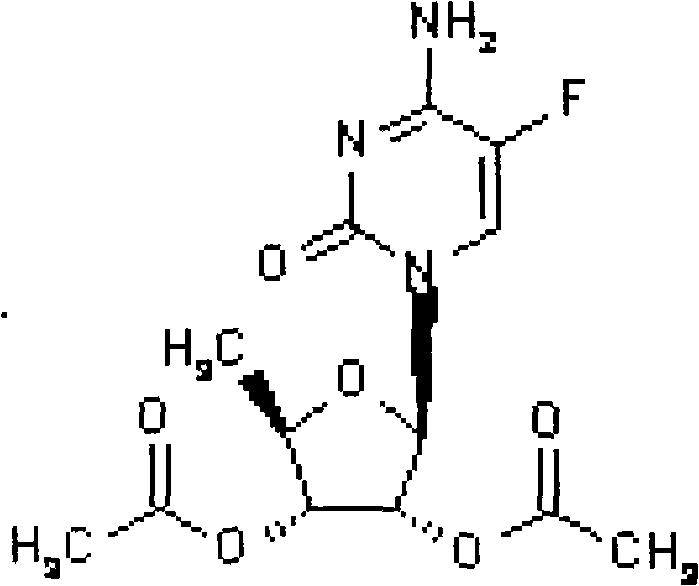
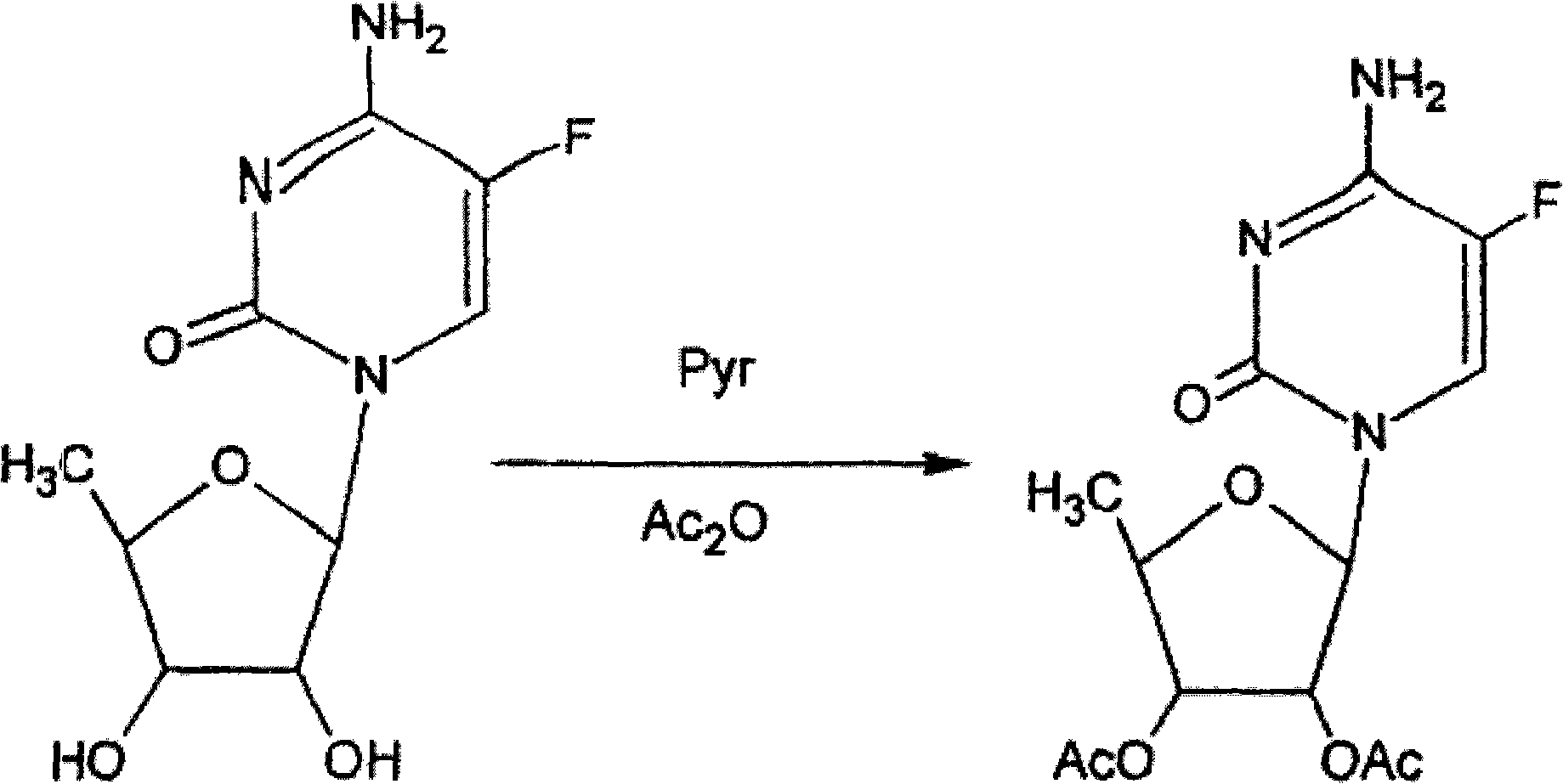
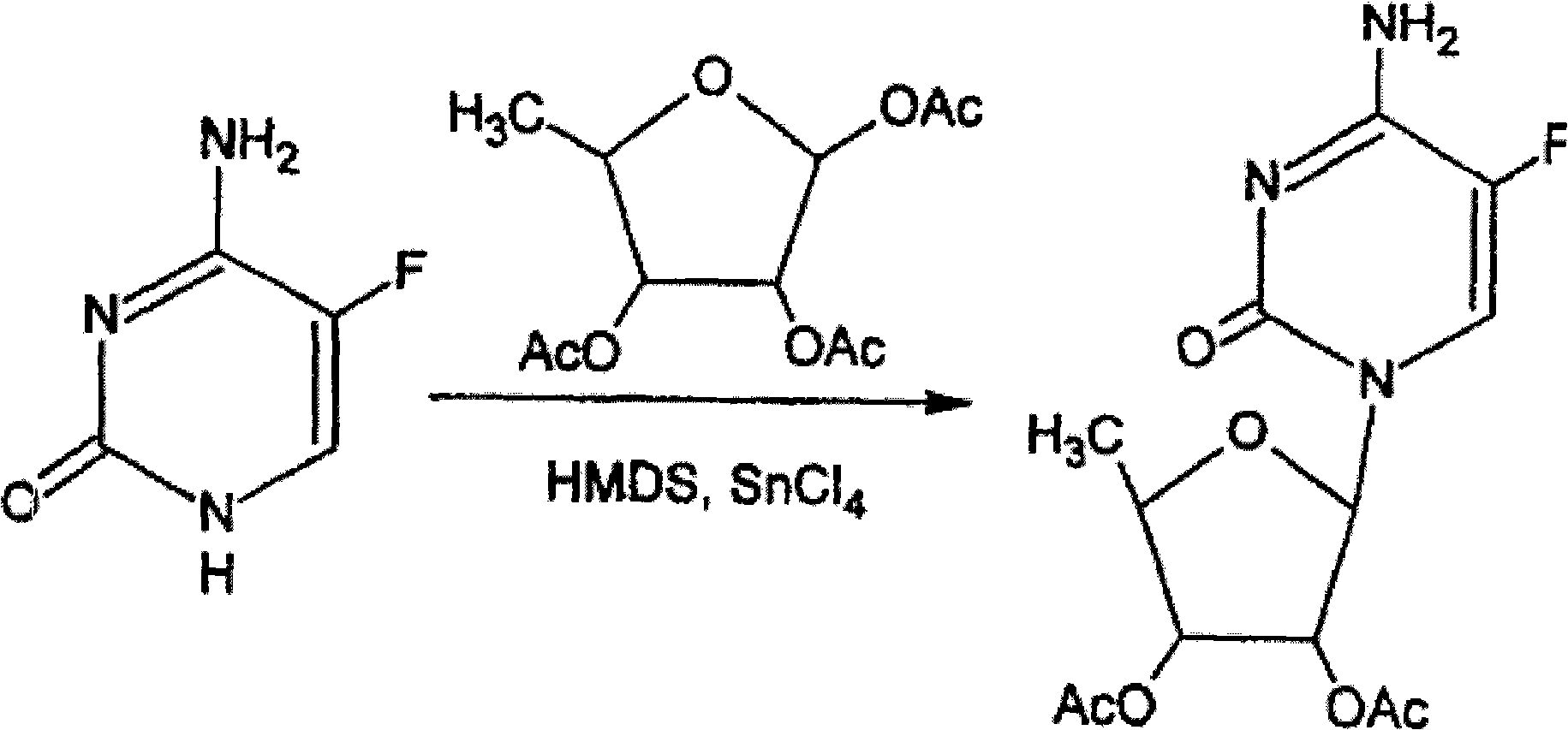
Preparation method of 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine
A technology of acetyl and flucytidine, which is applied to the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the cumbersome and complicated preparation methods of 5-deoxy-5-fluorocytidine, high production costs, and unnecessary Suitable for production conditions and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Dissolve 5-fluorocytosine (1.29g) in 30ml anhydrous toluene, cool to about 0°C, add trimethylsilyl trifluoromethanesulfonate (1.11g) and sodium iodide (3.0g) with stirring, The reaction was stirred for 30 minutes. Then, 5-deoxytriacetylribose (2.6g) was added, and the reaction was stirred at 0°C for 3 hours. After filtration, the filtrate was concentrated to dryness under reduced pressure, and dichloromethane and saturated sodium bicarbonate solution were added to the residue to extract. The aqueous layer was extracted with dichloromethane / methanol (10 / 1). The organic layers were combined and dried over anhydrous sodium sulfate . Filtered, concentrated to dryness under reduced pressure, and added 5 times the amount of isopropanol crystals to obtain 2',3'-Di-O-acetyl-5'-deoxy-5-fluorocytidine (2.67g, 81.1%), HPLC: 99.1%, mp 191.0-192.5°C.
Embodiment 2
[0021] Dissolve 5-fluorocytosine (1.29g) in 30ml anhydrous acetonitrile, cool to about 0°C, add trimethylsilyl trifluoromethanesulfonate (1.11g) and sodium iodide (3.0g) with stirring, The reaction was stirred for 30 minutes. Then, 5-deoxytriacetylribose (2.6g) was added, and the reaction was stirred at 0°C for 3 hours. After filtration, the filtrate was concentrated to dryness under reduced pressure, and dichloromethane and saturated sodium bicarbonate solution were added to the residue to extract. The aqueous layer was extracted with dichloromethane / methanol (10 / 1). The organic layers were combined and dried over anhydrous sodium sulfate . Filter, concentrate to dryness under reduced pressure, add 5 times the amount of isopropanol crystals to obtain 2',3'-Di-O-acetyl-5'-deoxy-5-fluorocytidine (2.60g, 79.0%), HPLC: 98.7%, mp 190.4-192.5°C.
Embodiment 3
[0023] Dissolve 5-fluorocytosine (1.29g) in 30ml of dry dichloromethane, cool to about 0℃, add trimethylsilyl trifluoromethanesulfonate (1.11g) and sodium iodide (3.0g) while stirring ), the reaction is stirred for 30 minutes. Then, 5-deoxytriacetylribose (2.6g) was added, and the reaction was stirred at 0°C for 3 hours. After filtration, the filtrate was concentrated to dryness under reduced pressure, and dichloromethane and saturated sodium bicarbonate solution were added to the residue to extract. The aqueous layer was extracted with dichloromethane / methanol (10 / 1). The organic layers were combined and dried over anhydrous sodium sulfate . Filter, concentrate to dryness under reduced pressure, add 5 times the amount of isopropanol crystals to obtain 2',3'-Di-O-acetyl-5'-deoxy-5-fluorocytidine (2.7g, 82.0%), HPLC: 99.2%, mp 191.5-192.5°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com