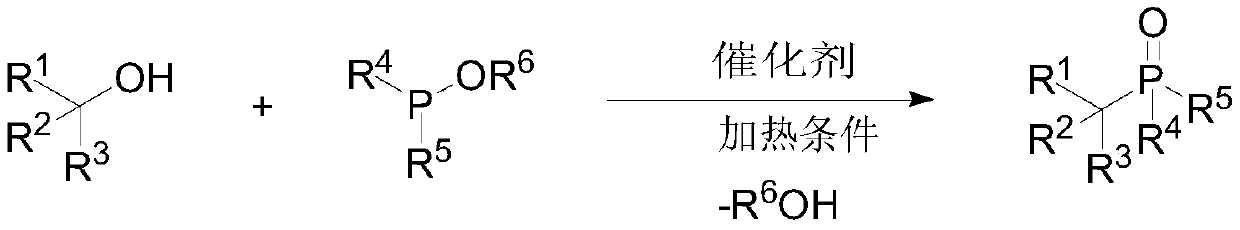
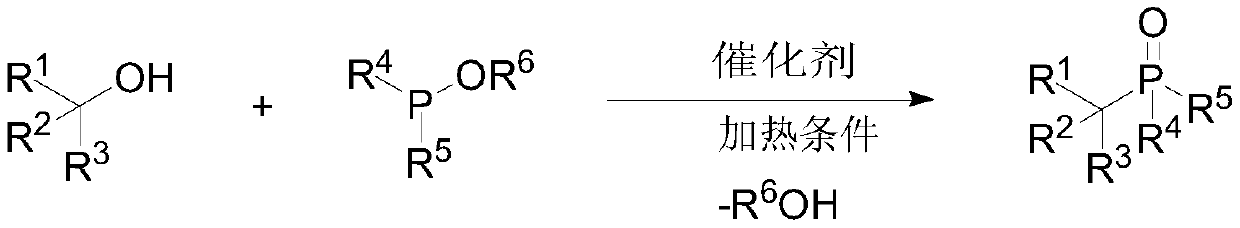
Preparation method of large-steric-hindrance alkyl substituted phosphite diester
A phosphinic acid diester and large steric hindrance technology, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve the problems that waste will pollute the environment and have little improvement. , to achieve the effect of easy operation and good theoretical research value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
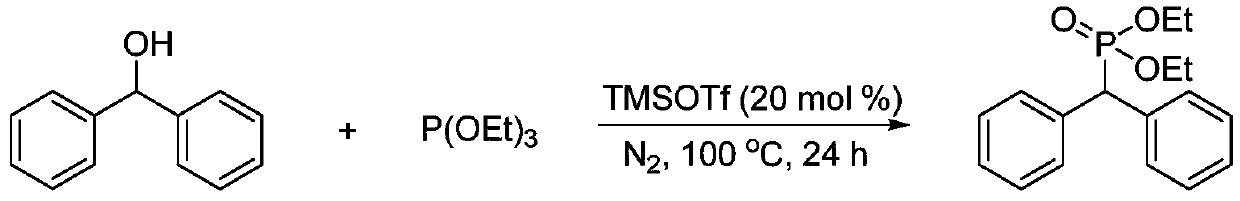
[0025] Synthesis of (diphenyl)methylphosphonite diethyl from benzhydryl alcohol and triethyl phosphite
[0026]
[0027] Add benzhydryl alcohol (0.0921g, 0.5mmol) in the tubular reactor, replace nitrogen three times, add triethyl phosphite (0.1715ml, 2.0equiv.), TMSOTf (0.0181ml, 20mol%) under the protection of nitrogen, Then react at 100°C for 24h under stirring. After the complete reaction was monitored by TLC, the product was separated and purified by column chromatography, and the separation yield was 82%. 1 H NMR (500MHz, CDCl 3 )δ7.53 (d, J = 7.0Hz, 4H), 7.29 (t, J = 7.0Hz, 4H), 7.25–7.17 (m, 2H), 4.43 (d, J = 25.0Hz, 1H), 4.12– 3.67(m,4H),1.09(t,J=6.9Hz,6H). 13 C NMR (126MHz, CDCl 3 )δ136.90(d, J=5.2Hz), 129.47(d, J=8.0Hz), 128.56, 127.11(d, J=1.6Hz), 62.62(d, J=7.0Hz), 51.35(d, J =138.2Hz), 16.22(d, J=5.8Hz). 31 P NMR (202MHz, CDCl 3 )δ25.10.
Embodiment 2
[0029] Synthesis of Diethyl (2-Methyldiphenyl)methylphosphonite from 2-Methylbenzhydryl Alcohol and Triethyl Phosphite
[0030]
[0031] Add 2-methylbenzhydrin (0.0991g, 0.5mmol) into the tubular reactor, replace the nitrogen three times, add triethyl phosphite (0.1715ml, 2.0equiv.), TMSOTf (0.0181ml, 20mol%), and then reacted at 100°C for 24h under stirring. After the complete reaction was monitored by TLC, the product was separated and purified by column chromatography, and the separation yield was 87%. 1 H NMR (500MHz, CDCl 3 )δ7.96(d, J=7.5Hz,1H),7.47(d,J=6.9Hz,2H),7.31–7.07(m,6H),4.67(d,J=26.0Hz,1H),4.04– 3.73(m,4H),2.32(s,3H),1.10(dt,J=17.0,7.0Hz,6H). 13 C NMR (126MHz, CDCl 3 )δ136.43(s), 136.37(d, J=6.9Hz), 135.34(d, J=3.8Hz), 130.61, 129.80(d, J=7.5Hz), 129.50(d, J=5.3Hz), 128.43, 127.12, 126.97, 126.20, 62.57(dd, J=15.9, 7.0Hz), 46.69(d, J=139.4Hz), 19.99, 16.23(t, J=5.1Hz). 31 P NMR (202MHz, CDCl 3 )δ25.95.
Embodiment 3
[0033] Synthesis of Diethyl (4-Methyldiphenyl)methylphosphonite from 4-Methylbenzhydryl Alcohol and Triethyl Phosphite
[0034]
[0035]Add 4-methylbenzhydrin (0.0991g, 0.5mmol) into the tubular reactor, replace the nitrogen three times, add triethyl phosphite (0.1715ml, 2.0equiv.), TMSOTf (0.0181ml, 20mol%), and then reacted at 100°C for 24h under stirring. After the completion of the reaction was monitored by TLC, the product was separated and purified by column chromatography, and the separation yield was 85%. 1 H NMR (500MHz, CDCl 3 )δ7.51(d, J=7.7Hz, 2H), 7.41(d, J=6.8Hz, 2H), 7.29(t, J=7.6Hz, 2H), 7.21(t, J=7.3Hz, 1H) ,7.11(d,J=7.9Hz,2H),4.39(d,J=25.1Hz,1H),4.03–3.76(m,4H),2.29(s,3H),1.11(q,J=7.2Hz, 6H). 13 C NMR (126MHz, CDCl 3 )δ137.15(d, J=5.0Hz), 136.69(d, J=2.0Hz), 133.87(d, J=5.2Hz), 129.45, 129.37(d, J=4.8Hz), 129.28(d, J = 2.8Hz), 128.52, 127.02 (d, J = 1.7Hz), 62.57 (dd, J = 6.9, 1.7Hz), 50.93 (d, J = 138.2Hz), 21.00, 16.24 (dd, J = 5.6, 3.3 Hz). 31 P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com