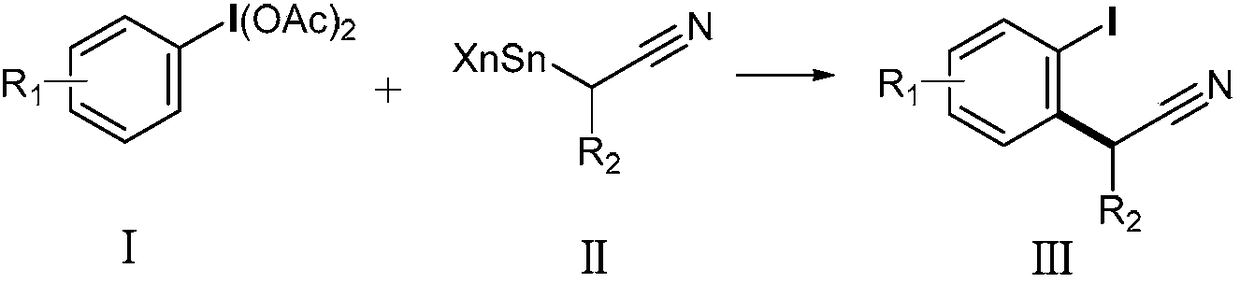
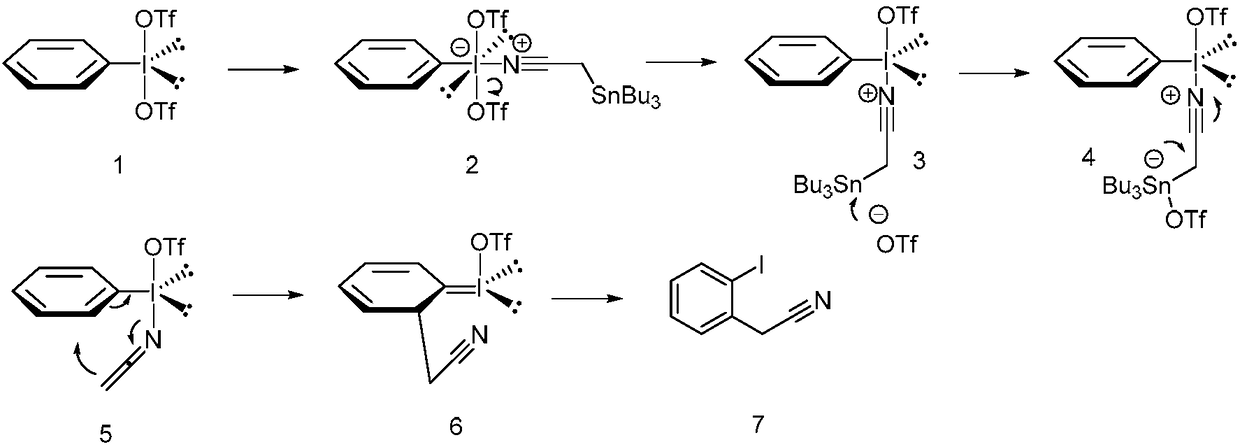
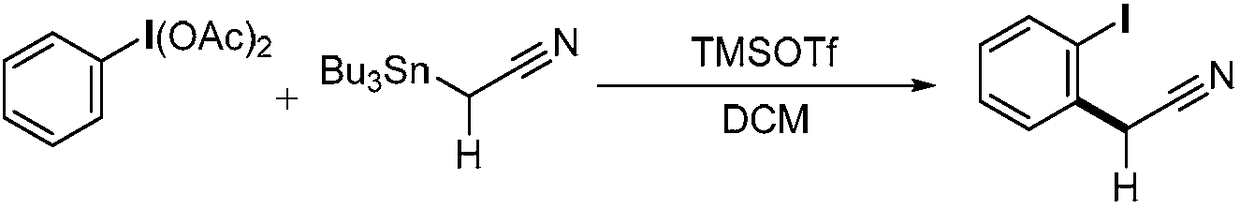Method for preparing alpha-aryl nitrile compound
A compound and aryl nitrile technology, applied in the field of preparing α-aryl nitrile compounds, can solve the problems of diversity of reaction substrates, limited scope of application, harsh reaction conditions, etc., and achieve easy products, mild reaction conditions and good selectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024] N 2 Under protection, redistilled dichloromethane (5 mL) was added to a 25 ml reaction tube, then 161 mg (0.5 mmol) of iodobenzene diacetate was added, and then 180 microliters of trimethylsilyl trifluoromethanesulfonate was added (TMSOTf, 1.0mmol), the reaction solution was stirred at room temperature for 5min, and finally 198mg (0.6mmol) of α-tributyltin acetonitrile was added at -78°C, stirred for 5min, and the reaction progress was tracked by thin-layer chromatography. After the reaction was completed, saturated Sodium bicarbonate solution (3ml) quenched the reaction, slowly warmed to room temperature, then extracted with dichloromethane (3mL×3), the organic phase was dried over anhydrous sodium sulfate, concentrated in vacuo, and separated by column chromatography (Rf=0.19 , Developing agent: petroleum ether / ethyl acetate=40 / 1, v / v), the obtained product α-aryl nitrile compound is a white solid, and the yield is 86%.
[0025] The target product is char...
Embodiment 2
[0029]
[0030] N 2 Under protection, redistilled dichloromethane (5mL) was added to a 25ml reaction tube, then 161mg (0.5mmol) of iodobenzene diacetate was added, and then 180 microliters of TMSOTf (1.0mmol) was added, and the reaction solution was heated at room temperature Stir at low temperature for 5min, and finally add 223mg (0.6mmol) of α-tributyltinvaleronitrile at -78°C, stir for 5min, track the reaction progress with thin-layer chromatography, add saturated sodium bicarbonate solution (3ml) to quench the reaction after the reaction , slowly warming up to room temperature, then extracting with dichloromethane (3mL×3), the organic phase was dried over anhydrous sodium sulfate, concentrated in vacuo, separated by column chromatography (Rf=0.33, developing solvent: petroleum ether / ethyl acetate =40 / 1, v / v), the obtained product α-aryl nitrile compound was a yellow oil, and the yield was 77%.
[0031] The target product is characterized as follows:
[0032] 1 H NMR ...
Embodiment 3
[0035]
[0036] N 2 Under protection, redistilled dichloromethane (5mL) was added to a 25ml reaction tube, then 161mg (0.5mmol) of iodobenzene diacetate was added, and then 180 microliters of TMSOTf (1.0mmol) was added, and the reaction solution was heated at room temperature Stir for 5 minutes, and finally add 252 mg (0.6 mmol) of α-tributyltin phenylpropionitrile at -78 ° C, stir for 5 minutes, track the reaction process with thin-layer chromatography, add saturated sodium bicarbonate solution (3 ml) after the reaction is completed to quench reaction, slowly warming to room temperature, and then extracting with dichloromethane (3mL×3), the organic phase was dried over anhydrous sodium sulfate, concentrated in vacuo, and separated by column chromatography (Rf=0.27, developing solvent: petroleum ether / ethyl acetate Ester=40 / 1, v / v), the product obtained was a yellow oil with a yield of 69%.
[0037] The target product is characterized as follows:
[0038] 1 H NMR (600MHz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com