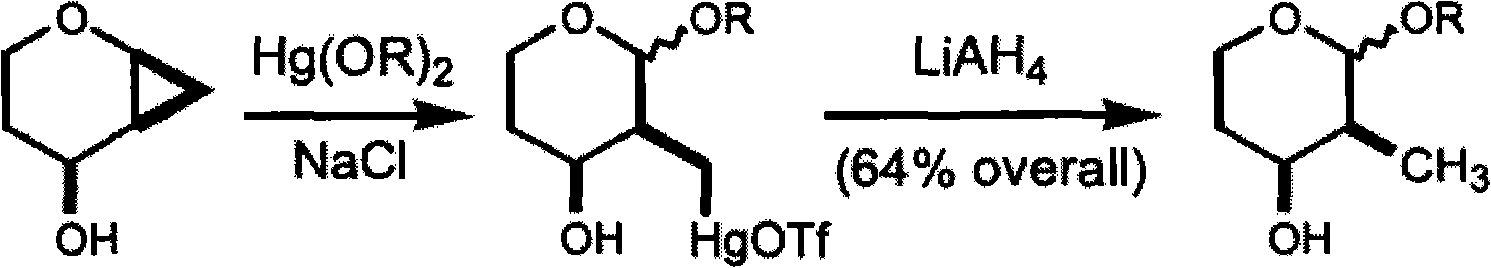
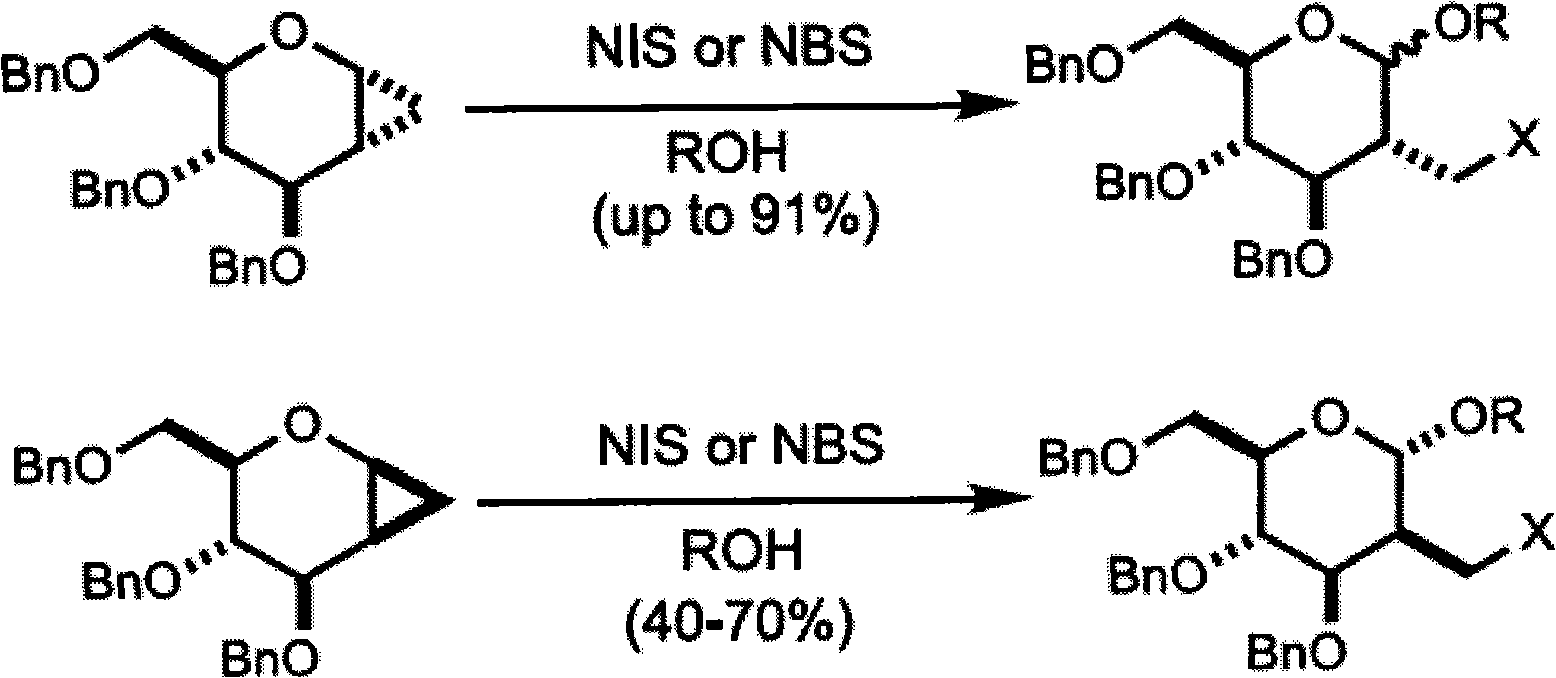
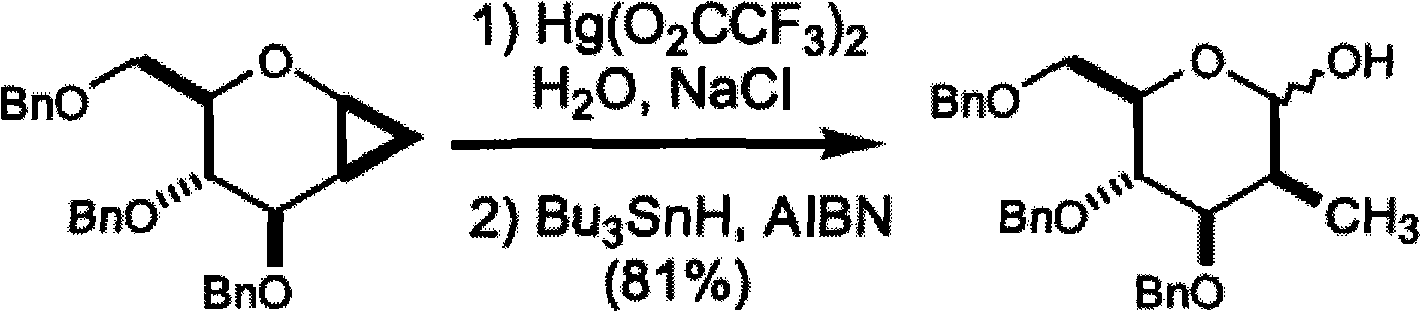Method for preparing 2-C-acetonyl-2-deoxy-D-galactopyranose and derivatives of 2-C-acetonyl-2-deoxy-D-galactopyranose
A technology for galactopyranosyl and galactopyranoside, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of high cost, rare reagents, environmental pollution, etc., and achieves low cost and high reaction efficiency. Short time and less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Under the protection of Ar, add 1,2-deoxy-3,4,6-tri-O-benzyl-7-C-acetyl-α-D-galactopyranose to a 5mL round bottom flask (47.2mg, 0.1mmol) and 1,2:3,4-di-O-isopropylidene-α-D-galactopyranose (28.2mg, 0.11mmol), then add dry dichloromethane (1mL) dissolved, then activated by adding Molecular sieves (47mg), stirred at room temperature for 30 minutes, the reaction temperature dropped to -20°C, added boron trifluoride ether (2.5μL, 0.02mmol), and then the temperature was slowly raised to react, and the reaction was detected by TLC until the end. Add an appropriate amount of triethylamine to quench the reaction, then add dichloromethane (5mL) to dilute the reaction system, filter, and pass the filtrate through water (10mL×2), saturated aqueous sodium bicarbonate (10mL×2) and saturated saline (10mL×2) 2) Washing and drying over anhydrous sodium sulfate. After filtration, the filtrate was concentrated and purified by silica gel column chromatography (eluent: petro...
Embodiment 2
[0055] Example 2: Under the protection of Ar, add 1,2-deoxy-3,4,6-tri-O-benzyl-7-C-acetyl-α-D-galactopyranose to a 5mL round bottom flask (47.2mg, 0.1mmol) and 1,2:3,4-di-O-isopropylidene-α-D-galactopyranose (28.2mg, 0.11mmol), then add dry ether (1mL) to dissolve, reactivation Molecular sieves (47mg) were stirred at room temperature for 30 minutes, the reaction temperature was lowered to -20°C, and boron trifluoride ether (2.5μL, 0.02mmol) was added, and then the temperature was slowly raised to react, and the reaction was detected by TLC until the end. Add an appropriate amount of triethylamine to quench the reaction, then add dichloromethane (5mL) to dilute the reaction system, filter, and filter the filtrate through water (10mL×2), saturated aqueous sodium bicarbonate (10mL×2) and saturated brine (10mL×2 ) and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated and purified by silica gel column chromatography (eluent: petroleum ether / eth...
Embodiment 3
[0056] Example 3: Under the protection of Ar, add 1,2-deoxy-3,4,6-tri-O-benzyl-7-C-acetyl-α-D-galactopyranose to a 5mL round bottom flask (47.2mg, 0.1mmol) and 1,2:3,4-di-O-isopropylidene-α-D-galactopyranose (28.2mg, 0.11mmol), followed by the addition of dry dichloromethane (1mL) dissolved, then added to activate Molecular sieves (47mg), stirred at room temperature for 30 minutes, the reaction temperature dropped to -20°C, trimethylsilyl trifluoromethanesulfonate (4.0μL, 0.04mmol) was added, and then the temperature was slowly raised to react, and the reaction was detected by TLC until the end. Add an appropriate amount of triethylamine to quench the reaction, then add dichloromethane (5mL) to dilute the reaction system, filter, and pass the filtrate through water (10mL×2), saturated aqueous sodium bicarbonate (10mL×2) and saturated brine (10mL×2 ) and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated and purified by silica gel column chr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com