Preparation method of Cangrelor intermediate
A technology for intermediates and compounds is applied in the field of preparation of cangrelor intermediate 6-N-ethyl-2-thio)adenosine, and can solve the problems of low yield, high price, unfavorable industrial production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
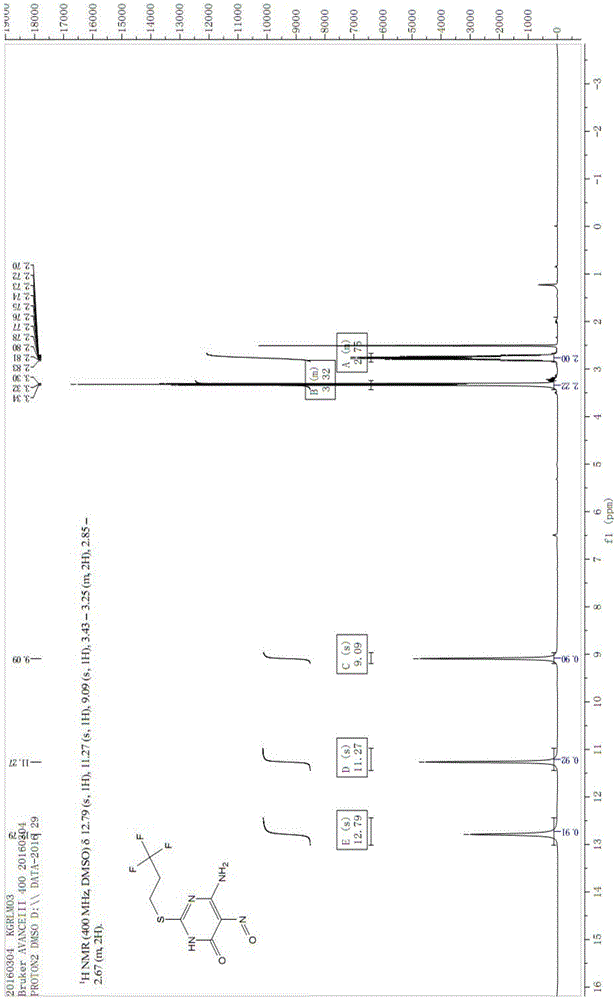
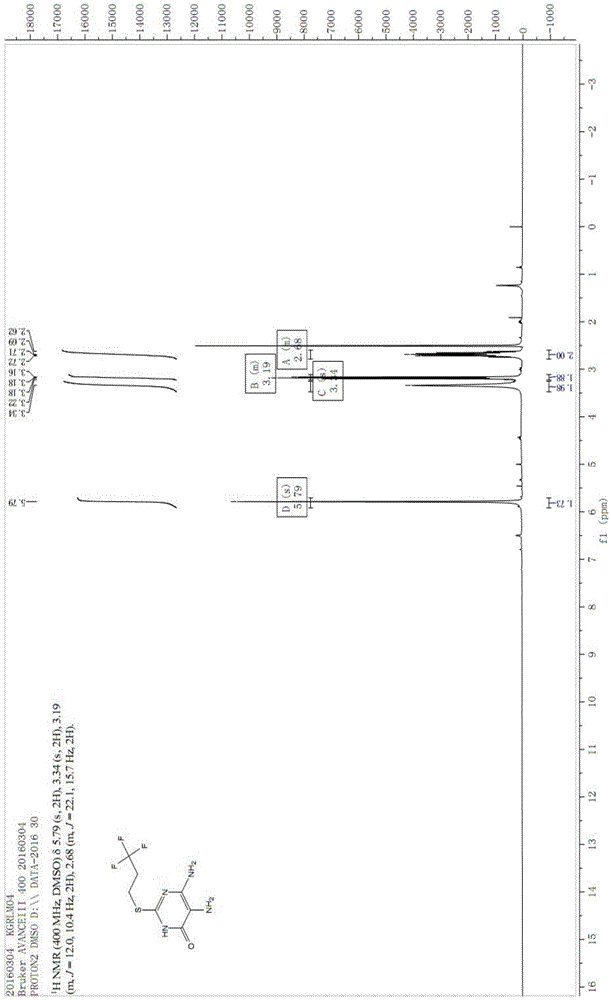
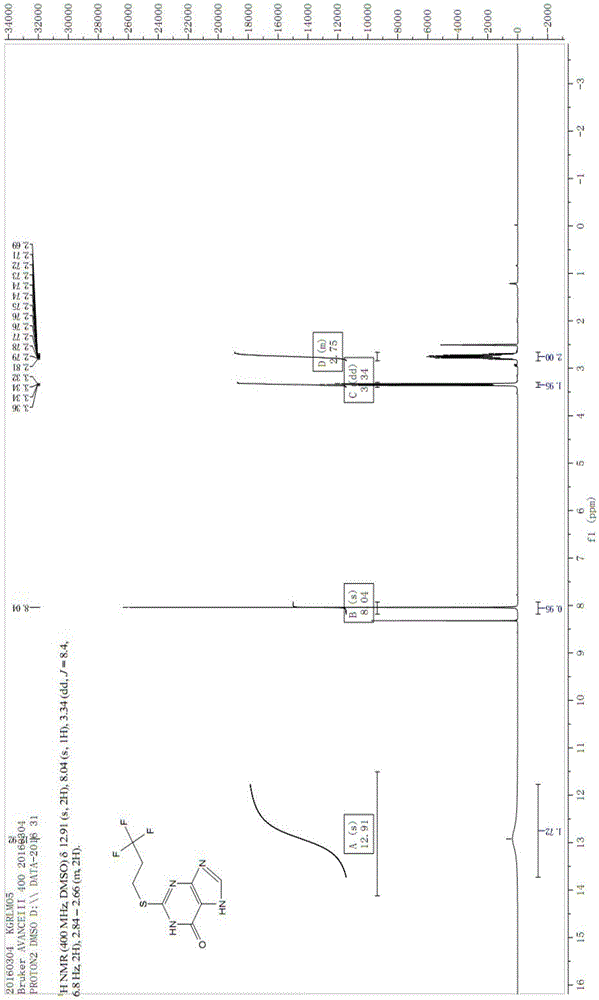
Image
Examples
Embodiment 1
[0081] A preparation method of 6-N-(2-(methylthio)ethyl-2-((3,3,3-trifluoropropyl)thio)adenosine (I), comprising the following steps:
[0082] (1) Preparation of compound 16
[0083] At room temperature, take by weighing sodium ethoxide 127.8g ((1.88mol) and join the 3L reaction flask of 670g ethanol, stir to dissolve all, add thiourea 67.3g (0.884mol). Heat to 45°C, weigh ethyl cyanoacetate 100 g (0.884 mol) of ester was added to the reaction flask and heated to reflux. After 2 h of reflux, TLC reaction was completed, and the temperature was lowered to 15 ° C. Filtered, the filter cake was washed once with 200 g of absolute ethanol, the filter cake was taken out, and added to a 3L reaction flask, Add water 1kg and stir to make it dissolve, add acetic acid below 20 ° C, adjust pH=4, a large amount of solid is separated out. Suction filtration. Filter cake is dried overnight at 46 ° C to obtain product 116.4g, yield 92%.
[0084] (2) Preparation of compound 17
[0085] At roo...
Embodiment 2
[0104] (1) At room temperature, weigh 103.2 g (0.92 mol) of potassium tert-butoxide into a 1 L reaction flask of 450 g of methanol, stir to dissolve it completely, and add 30.4 g (0.4 mol) of thiourea. Heated to 45°C, weighed 47.43 g (0.42 mol) of ethyl cyanoacetate into the reaction flask and heated to reflux. After refluxing for 2.7 hours, the TLC reaction was completed, lowered to 15°C, filtered, and the filter cake was washed once with 116 g of methanol, Take out the filter cake, add it into a 1L reaction flask, add 570 g of water and stir to dissolve it, add 15% hydrochloric acid below 20°C, adjust pH=4, and a large amount of solids are precipitated. Suction filtration. The filter cake was dried at 45°C overnight to obtain 49.97 g of product with a yield of 87.5%.
[0105] (2) Preparation of compound 17
[0106] At room temperature, weigh 190 g (1.4 mol) of DBU and add it to a 2L three-necked flask, add 301 g of DMSO and stir, add 43 g (0.3 mol) of compound 16, start st...
Embodiment 3
[0122](1) At room temperature, weigh 159.2 g (1.42 mol) of triethylamine and add it to a 2L reaction flask of 750 g of methanol, stir to dissolve it completely, and add 45 g (0.59 mol) of thiourea. Heated to 45°C, weighed 68.2g (0.6mol) of ethyl cyanoacetate into the reaction flask and heated to reflux. After refluxing for 2.5h, the TLC reaction was completed, lowered to 15°C, filtered, and the filter cake was washed with 190g of isopropanol. Once, take out the filter cake, add it into a 2L reaction flask, add 810g of water and stir to dissolve it, add 40% formic acid below 20°C, adjust pH=4, and a large amount of solid is precipitated. Suction filtration. The filter cake was dried at 45°C overnight to obtain 76 g of product with a yield of 89.9%.
[0123] (2) Preparation of compound 17
[0124] At room temperature, weigh 32.83g (1.37mol) of lithium hydroxide and add it to a 2L there-necked flask, add 588g of DMF and stir, add 70g (0.49mol) of compound, start stirring, and a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com