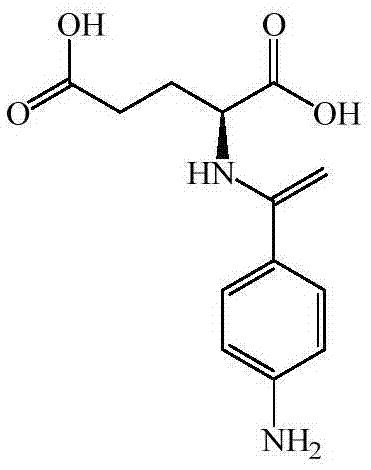
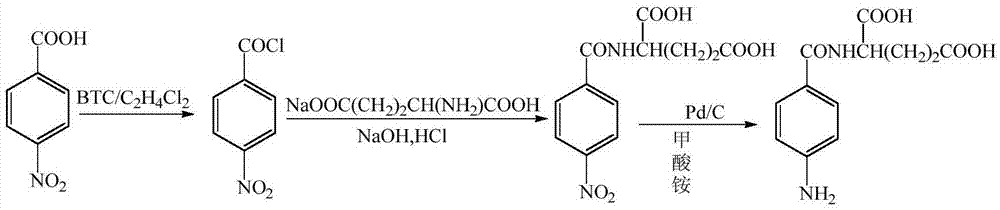
Preparation method of N (4-aminobenzoyl)-L-glutamic acid
A technology of p-aminobenzoyl and p-nitrobenzoic acid, applied in the field of preparation of N-p-aminobenzoyl-L-glutamic acid, which can solve the inconvenience of industrial production, unsatisfactory yield, and unfavorable environmental protection, etc. problems, to achieve the effect of easy operation, mild reaction and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Preparation of ethylene dichloride solution of p-nitrobenzoyl chloride
[0026] Add 16.71g (0.1mol) of p-nitrobenzoic acid, 67g of dichloroethane, and 0.29g (0.004mol) of DMF into a reaction vessel with a tail gas absorption device, raise the temperature to 40°C, and slowly add BTC / C 2 h 4 Cl 2 Solution 15.5ml, dropwise reflux reaction for 1 hour, concentrated under reduced pressure to about 1 / 3 of the original volume, to recover the solvent and remove hydrogen chloride, the residue is 32g of dichloroethane solution of p-nitrobenzoyl chloride, HPLC The purity of the target substance was determined to be 57.81%.
[0027] (2) Preparation of N-p-nitrobenzoyl-L-glutamic acid
[0028] Add 18.73 g (0.11 mol) of sodium glutamate and 75 g of water into the reaction vessel, adjust the pH to 8 with 1 mol / L aqueous sodium hydroxide solution under stirring, and slowly add 28.31 g of the mixture obtained in step (1) dropwise at 0°C. The dichloroethane solution of nitrobenzoy...
Embodiment 2
[0032] (1) Preparation of ethylene dichloride solution of p-nitrobenzoyl chloride
[0033] Add 16.71g (0.1mol) of p-nitrobenzoic acid, 83.6g of dichloroethane, and 0.22g (0.003mol) of DMF into a reaction vessel with a tail gas absorption device, raise the temperature to 42°C, and slowly add BTC / C 2 h 4 Cl 2 Solution 10ml, dropwise reflux reaction for 1.5 hours, concentrated under reduced pressure to about 1 / 3 of the original volume, to recover the solvent and remove hydrogen chloride, the residue is 31.1g of dichloroethane solution of p-nitrobenzoyl chloride, HPLC The purity of the target substance was determined to be 58.56%.
[0034] (2) Preparation of N-p-nitrobenzoyl-L-glutamic acid
[0035] Add 18.73 g (0.11 mol) of sodium glutamate and 75 g of water into the reaction vessel, adjust the pH to 8.5 with 1 mol / L aqueous sodium hydroxide solution under stirring, and slowly add 29.7 g of the mixture obtained in step (1) dropwise at 3°C. The dichloroethane solution of nit...
Embodiment 3
[0039] (1) Preparation of ethylene dichloride solution of p-nitrobenzoyl chloride
[0040] Add 16.71g (0.1mol) of p-nitrobenzoic acid, 100.3g of dichloroethane, and 0.37g (0.005mol) of DMF into a reaction vessel with a tail gas absorption device, raise the temperature to 45°C, and slowly add BTC / C 2 h 4 Cl 2 Solution 12ml, dropwise reflux reaction for 2 hours, concentrated under reduced pressure to about 1 / 3 of the original volume, to recover the solvent and remove hydrogen chloride, the residue is 32g of dichloroethane solution of p-nitrobenzoyl chloride, determined by HPLC The purity of the target object was 57.23%.
[0041] (2) Preparation of N-p-nitrobenzoyl-L-glutamic acid
[0042] Add 18.73 g (0.11 mol) of sodium glutamate and 75 g of water into the reaction vessel, adjust the pH to 9 with 1 mol / L aqueous sodium hydroxide solution under stirring, and slowly add 29.7 g of the mixture obtained in step (1) dropwise at 5°C. The dichloroethane solution of nitrobenzoyl chl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com