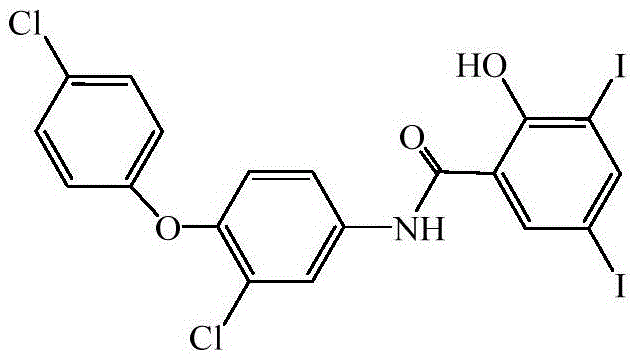
A preparing method of rafoxanide
A technology of iodoxanamide and chlorophenyl ether, which is applied in the field of preparation of iodoxanamide, can solve the problems of inconvenient industrial production, unsatisfactory yield, long reaction time, etc., and achieves easy operation, mild reaction and excellent reaction conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation of embodiment 1 iodoxanamide
[0026] (1) Preparation of 2-chloro-4-nitrophenyl-p-chlorophenyl ether
[0027] Add 25.71g (0.2mol) of p-chlorophenol and 77.13g of DMF to the reaction vessel, add 11.45g (0.204mol) of potassium hydroxide under stirring, react at 35°C for 20 minutes, and add 38.4g of 3,4-dichloronitrobenzene (0.2mol), catalyzer cuprous chloride 0.396g (0.004mol), be warming up to reflux temperature, react 5 hours, reaction solution is cooled to room temperature, filter and remove insoluble impurity, filtrate underpressure distillation reclaims solvent, add water 100ml stirring under room temperature, Ocher needle-like crystals were precipitated, filtered and dried to obtain 55.4 g of 2-chloro-4-nitrophenyl-p-chlorophenyl ether, with an HPLC purity of 98.52% and a yield of 96.1% (based on p-chlorophenol).
[0028] (2) Preparation of 4-amino-2-chlorophenyl-p-chlorophenyl ether
[0029] Add 28.4g (0.1mol) of 2-chloro-4-nitrophenyl-p-chlorophe...
Embodiment 2
[0034] Preparation of embodiment 22-chloro-4-nitrophenyl-p-chlorophenyl ether
[0035]Add 25.71g (0.2mol) of p-chlorophenol and 102.84g of DMF to the reaction vessel, add 11.76g (0.21mol) of potassium hydroxide under stirring, react at 38°C for 30 minutes, and add 38.4g of 3,4-dichloronitrobenzene (0.2mol), catalyzer cuprous chloride 0.594g (0.006mol), be warming up to reflux temperature, react 5.5 hours, reaction solution is cooled to room temperature, filter and remove insoluble impurity, filtrate underpressure distillation reclaims solvent, add water 100ml stirring under room temperature, Precipitated khaki needle-like crystals, filtered and dried to obtain 53.8 g of 2-chloro-4-nitrophenyl-p-chlorophenyl ether, with an HPLC purity of 97.34% and a yield of 92.2% (based on p-chlorophenol).
Embodiment 3
[0036] Preparation of Example 32-chloro-4-nitrophenyl-p-chlorophenyl ether
[0037] Add 25.71g (0.2mol) of p-chlorophenol and 128.55g of DMF into the reaction vessel, add 12.32g (0.22mol) of potassium hydroxide under stirring, react at 40°C for 40 minutes, and add 38.4g of 3,4-dichloronitrobenzene (0.2mol), catalyzer cuprous chloride 0.792g (0.008mol), be warming up to reflux temperature, react 6 hours, reaction solution is cooled to room temperature, filter and remove insoluble impurity, filtrate underpressure distillation reclaims solvent, add water 100ml and stir under room temperature, Ocher needle crystals were precipitated, filtered and dried to obtain 54.1 g of 2-chloro-4-nitrophenyl-p-chlorophenyl ether, with an HPLC purity of 97.80% and a yield of 93.67% (calculated as p-chlorophenol).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com