Lenalidomide intermediate preparation method
A compound and better technology, applied in the field of synthesis of chemical drugs, can solve problems such as large environmental impact, difficulty in labor protection for experimenters, and difficulty in environmentally sound treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The preparation method of the present invention comprises the steps of:
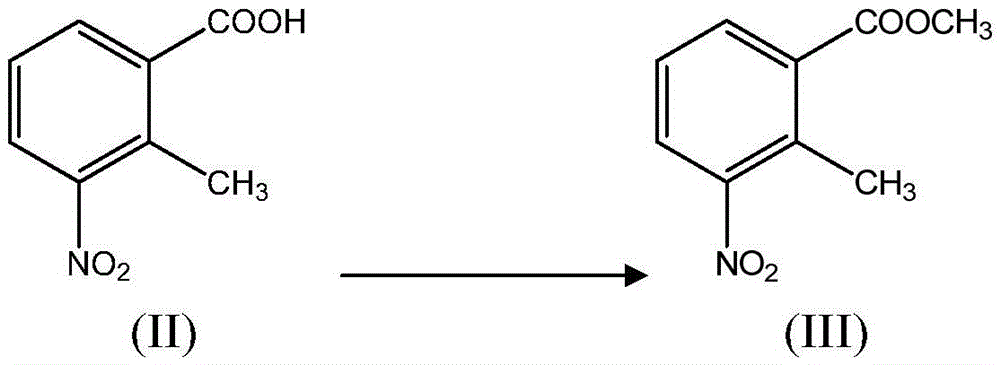
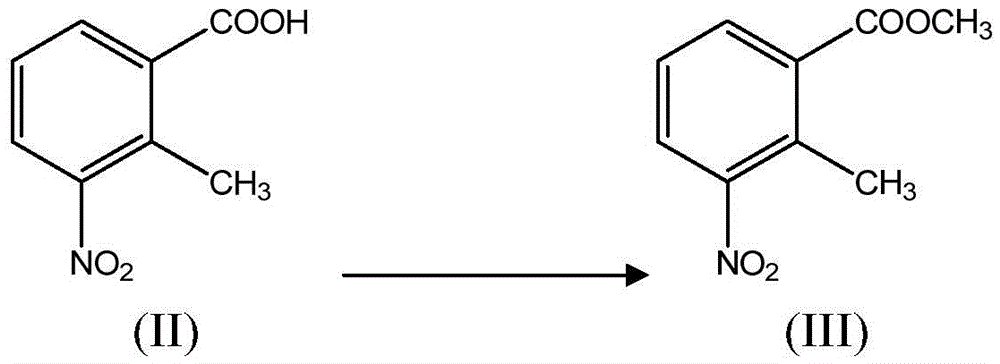
[0043] (i) in the presence of a catalyst, the compound of formula II is reacted with methanol to obtain the compound of formula III;
[0044]
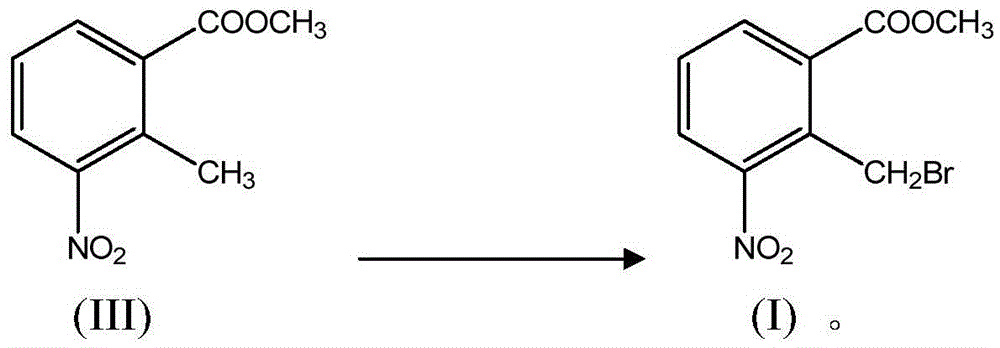
[0045] (ii) in an inert solvent, in the presence of a free radical initiator, the compound of formula III reacts with a brominating reagent to prepare the compound of formula I
[0046]
[0047]In another preferred example, the compound of formula II is suspended in methanol, and thionyl chloride is added dropwise with stirring, and heated to reflux for 2 hours after the dropwise addition is completed. The resulting reactant was poured into water, stirred, and an off-white solid was precipitated. After filtering, the filter cake was washed with water and dried under reduced pressure to obtain methyl 2-methyl-3-nitrobenzoate (III).
[0048]
[0049] In another preferred example, 2-methyl-3-nitrobenzoic acid methyl ester (III), N-bromosuccinimide ...
Embodiment 1
[0065] 1-1. Preparation of compound of formula III
[0066] Suspend the compound of formula II (40.0 g, 0.22 mol) in methanol (500 ml), add thionyl chloride (32.6 ml, 0.44 mol) dropwise under stirring, and heat to reflux for 2 h after the dropwise addition. The obtained reactant was poured into water (500ml), stirred, and an off-white solid was precipitated. After filtering, the filter cake was washed with water and dried under reduced pressure to obtain methyl 2-methyl-3-nitrobenzoate (III) (42 g), mp63-65°C (98% yield, mp61-63°C).
[0067] 1 HNMR (CDCl 3 )δ:2.63(s,3H,CH 3 ),3.95(s,3H,OCH 3 ), 7.39 (t, 1H, J = 7.9Hz, Ph), 7.85 (d, 1H, J = 7.9Hz, Ph), 8.00 (d, 1H, J = 7.9Hz, Ph). EI-MS (m / z): 195 (M+), 178, 165, 89.
[0068] 1-2 Formula I compound preparation
[0069] Add formula III compound (30g, 0.15mol), N-bromosuccinimide (NBS) (48g, 0.27mol) and azobisisobutyl Nitrile (AIBN) (3.1ml, 0.021mol), 1,2-dichloroethane (C 2 h 4 Cl 2 ) (100ml), stir and mix evenly, he...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com