Method for preparing 2-(substituted phenyl) methylamino-3-nitrobenzene methyl formate by one-pot method
A technology of methyl tert-butoxycarbonylaminobenzoate and methyl nitrobenzoate, applied in the fields of chemical industry and chemical medicine, can solve the problems of high solvent consumption, large waste water pollution, low yield and the like, and achieves improved operating environment, The effect of reducing labor intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
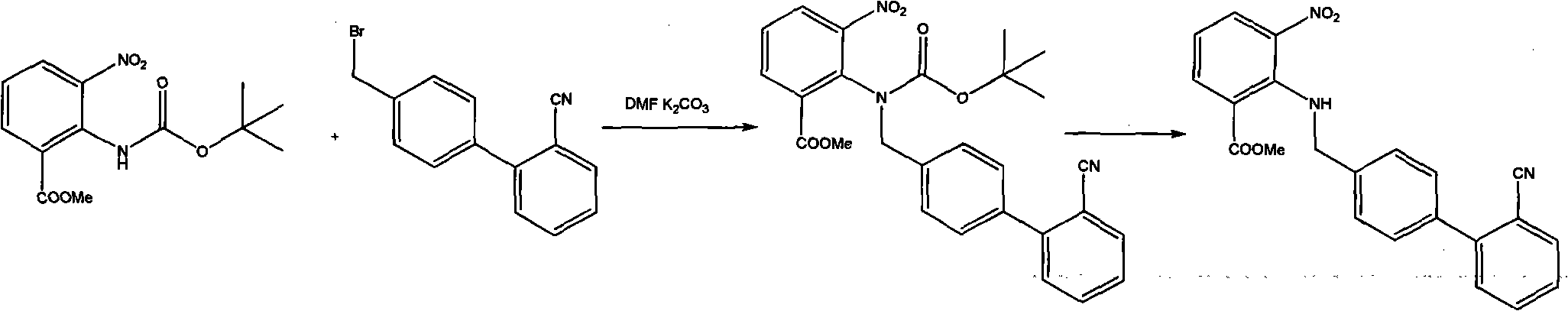
[0042] Example 1. Preparation of methyl 2-[[(2'-cyanobiphenyl)-4-yl]methyl]amino-3-nitrobenzoate
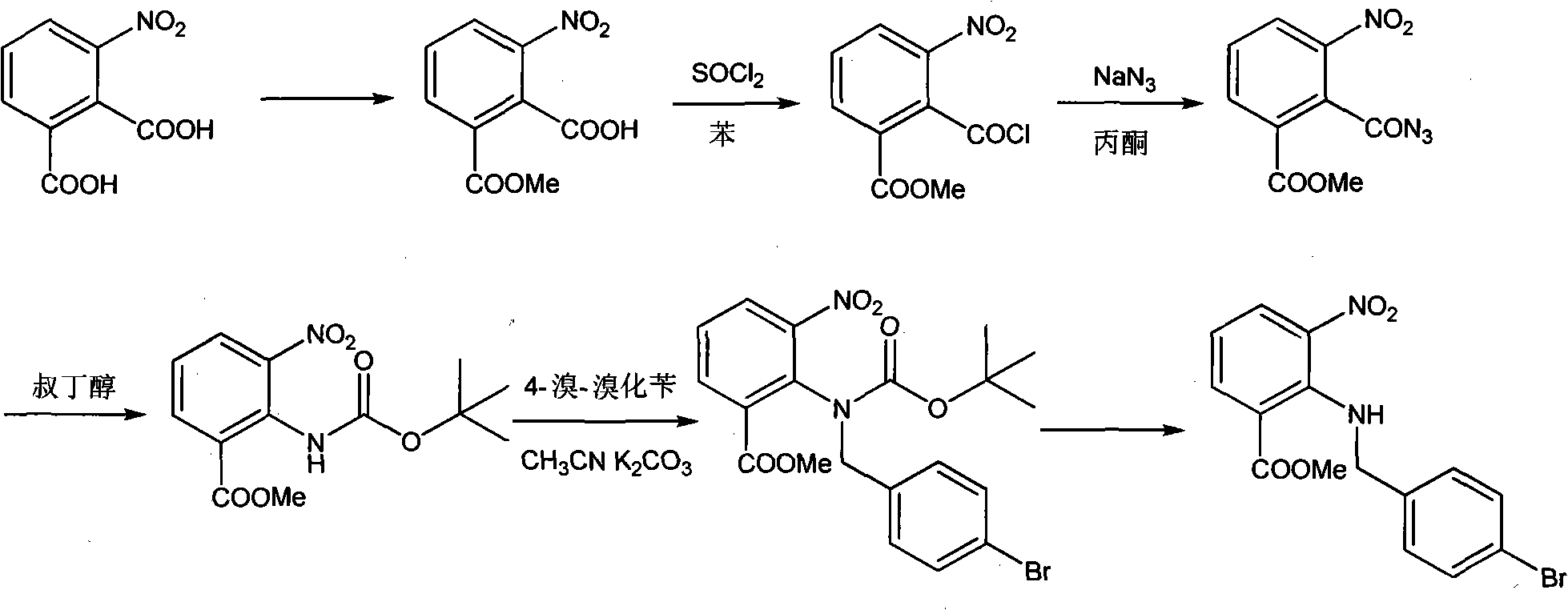
[0043] Add 90g of methyl 3-nitro-2-carboxybenzoate and 150ml of dimethylformamide (DMF) into a 1000ml three-necked flask, add 70g of thionyl chloride dropwise in a water bath, stir at room temperature for 3 hours after dropping, add 250ml of chloroform, Tetrabutylammonium bromide 4g, stir to dissolve. t3 20% NaOH solution was added dropwise to neutrality, and when t<20°C, the layers were separated, and the aqueous layer was extracted with 50 ml of chloroform. Add 100ml of tert-butanol to the organic layer, slowly raise the temperature, reflux at 65-70°C for 2h, cool to t<30°C, add 400ml of water to wash, stand to separate the layers, extract the aqueous layer with 100ml of chloroform, and combine the organic layers. Add 76.2g of 4-bromomethyl-2′-cyanobiphenyl and 4g of tetrabutylammonium bromide to the organic layer, stir to dissolve, add 60g of 30% NaOH dropwise at t<10°C, stir...
example 2
[0044] Example 2. Preparation of methyl 2-[[(2'-cyanobiphenyl)-4-yl]methyl]amino-3-nitrobenzoate
[0045]Add 90g of methyl 3-nitro-2-carboxybenzoate and 150ml of dimethylformamide (DMF) into a 1000ml three-necked flask, add 70g of thionyl chloride dropwise in a water bath, stir at room temperature for 3 hours after dropping, add 250ml of chloroform, 4g 15-crown (ether)-5, stirred to dissolve. t3 20% NaOH solution was added dropwise to neutrality, and when t<20°C, the layers were separated, and the aqueous layer was extracted with 50 ml of chloroform. Add 100ml of tert-butanol to the organic layer, slowly raise the temperature, reflux at 65-70°C for 2h, cool to t<30°C, add 400ml of water to wash, stand to separate the layers, extract the aqueous layer with 100ml of chloroform, and combine the organic layers. Add 76.2g of 4-bromomethyl-2′-cyanobiphenyl and 4g of tetrabutylammonium bromide to the organic layer, stir to dissolve, add 60g of 30% NaOH dropwise at t<10°C, stir at 25...
example 3
[0046] Example 3. Preparation of methyl 2-(4'-bromophenyl)methylamino-3-nitrobenzoate
[0047] Add 90g of methyl 3-nitro-2-carboxybenzoate and 150ml of DMF to a 1000ml three-necked flask, add 70g of thionyl chloride dropwise in a water bath, stir at room temperature for 3h after dropping, add 250ml of chloroform, 4g of tetrabutylammonium bromide , stir to dissolve. t3 20% NaOH solution was added dropwise to neutral, the layers were separated at <20°C, and the aqueous layer was extracted with 50ml of chloroform. The organic layer was washed once more with 400ml of water (t<20°C). The organic layer was dried with anhydrous magnesium sulfate, the desiccant was filtered off, 100ml of tert-butanol was added, the temperature was raised slowly, and the mixture was refluxed at 65-70°C for 2h. Cool to t<30°C, add 400ml of water to wash, let stand to separate the layers, extract the aqueous layer with 100ml of chloroform, and combine the organic layers. Add 70g of p-bromobenzyl bromi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com