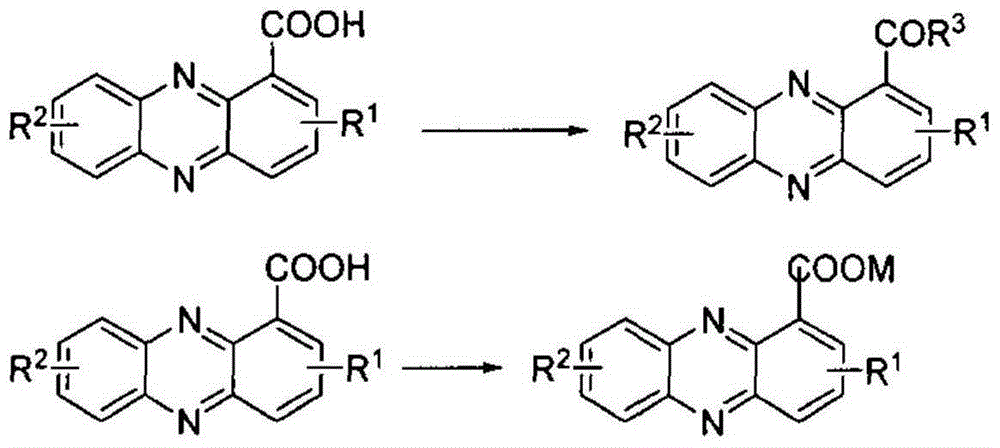
Preparation method for phenazine-1-carboxylic acid
A technology of carboxylic acid and phenazine, which is applied in the field of preparation of active components of pesticides, can solve the problems of large investment in equipment, long reaction steps, and difficult availability of raw materials, etc., and achieve easy-to-obtain reaction raw materials, mild reaction conditions, and easy post-processing Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
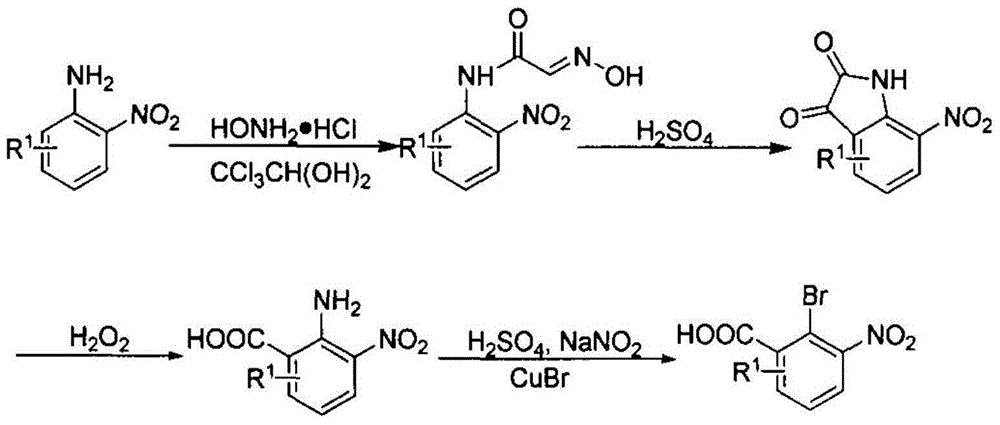
[0039] Preparation of α-oximinoacetanilide
[0040] Add 30g of chloral hydrate, 70g of hydroxylamine hydrochloride, 22g of anhydrous sodium sulfate, and 800g of water into a 1000mL four-necked bottle in sequence, and heat to 65°C. The suspension (20 g of 2-nitroaniline, 20 mL of 2N hydrochloric acid) was added. Insulation reaction 20h. Cool down to 5-8°C and keep warm for 2h. Filtration gave a yellow solid. After drying, 25 g of the title compound was obtained.
[0041] Preparation of isatin
[0042] Add 137g of concentrated sulfuric acid into a 100mL three-necked flask and heat to 80°C. Add 25 g of α-oximinoacetanilide several times, and control the temperature not to exceed 90°C. After the addition was complete, the reaction was incubated at 90°C for 2h. After cooling down to room temperature, the reaction solution was poured into about 300 g of ice to quench the reaction. Insulate at 5°C for 2 hours, and filter to obtain a khaki product. After drying, 24 g of the t...
Embodiment 2
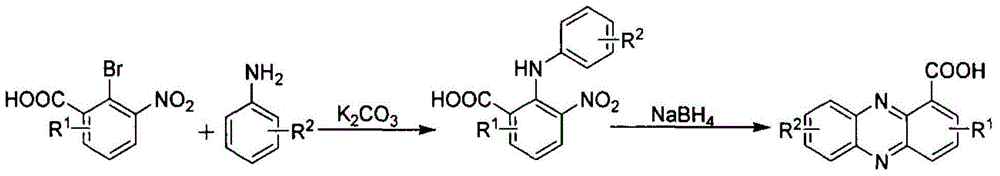
[0054] A method for preparing phenazine-1-carboxylic acid, using the following steps:
[0055] (1) Synthesis of 2-bromo-3 nitrobenzoic acid
[0056] The reaction of aniline with chloral hydrate and hydroxylamine produces α-oximinoacetanilide, which is then treated with concentrated sulfuric acid to obtain isatin, which is reacted with hydrogen peroxide to obtain 2-amino-3-nitrobenzoic acid, which is then subjected to Sandmeyer reaction to obtain 2-bromo -3 nitrobenzoic acid, specifically adopt the following steps:
[0057] Mix chloral hydrate, hydroxylamine hydrochloride, and anhydrous sodium sulfate in a weight ratio of 30:80:30 and dissolve them in water, heat to 65°C, add 2-nitroaniline suspension, 2-nitroaniline The weight ratio to chloral hydrate is 30:30, keep the temperature down to 5-8°C after 20 hours of heat preservation, keep heat for 2 hours, and filter to obtain a yellow solid that is α-oximinoacetanilide.
[0058] The prepared α-oximino acetanilide was mixed wi...
Embodiment 3
[0067] A method for preparing phenazine-1-carboxylic acid, using the following steps:
[0068] (1) Synthesis of 2-bromo-3 nitrobenzoic acid
[0069] The reaction of aniline with chloral hydrate and hydroxylamine produces α-oximinoacetanilide, which is then treated with concentrated sulfuric acid to obtain isatin, which is reacted with hydrogen peroxide to obtain 2-amino-3-nitrobenzoic acid, which is then subjected to Sandmeyer reaction to obtain 2-bromo -3 Nitrobenzoic acid. Specifically take the following steps:
[0070] Mix chloral hydrate, hydroxylamine hydrochloride, and anhydrous sodium sulfate in a weight ratio of 20:60:20 and dissolve them in water, heat to 65°C, add 2-nitroaniline suspension, 2-nitroaniline The weight ratio to chloral hydrate is 20:20, heat preservation reaction for 20 hours, then cool down to 5°C, heat preservation for 2 hours, and filter to obtain a yellow solid which is α-oximinoacetanilide;
[0071] The prepared α-oximino acetanilide was mixed w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com