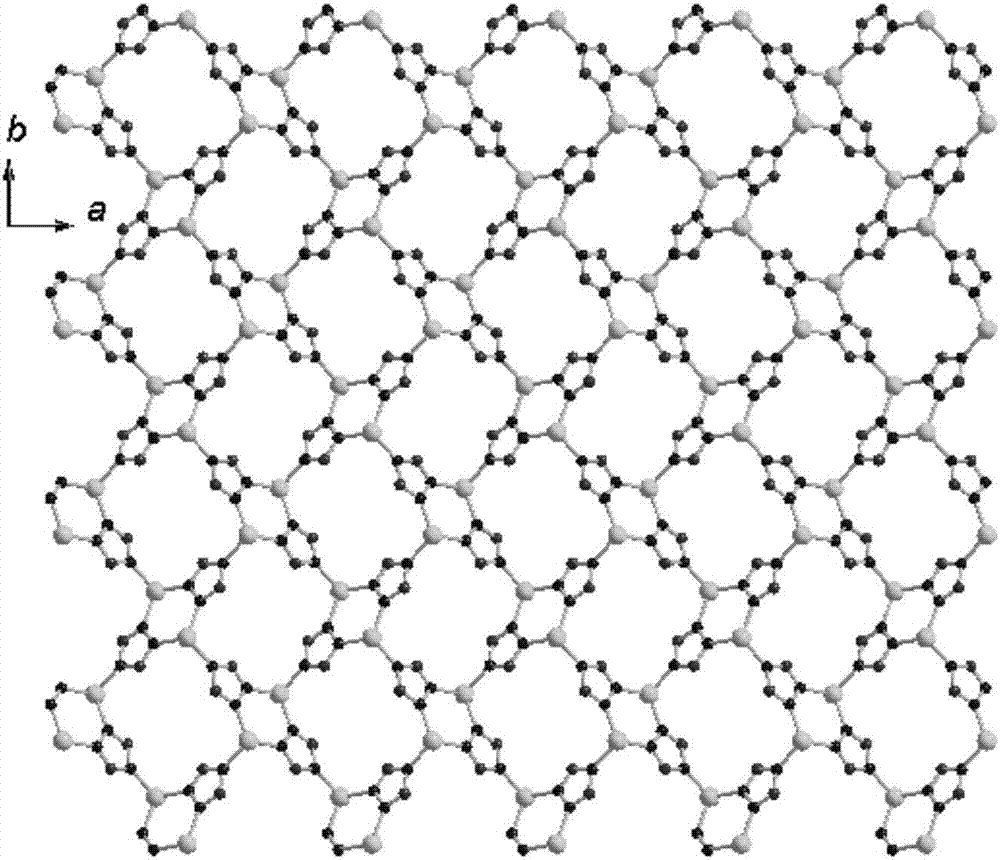
Two-dimensional zinc coordination polymer and preparation method thereof
A zinc coordination polymer, two-dimensional layered technology, applied in the field of coordination polymer preparation, can solve the problems of inconvenient detection and expensive detection instruments, and achieve the effect of good fluorescence recognition and sensing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
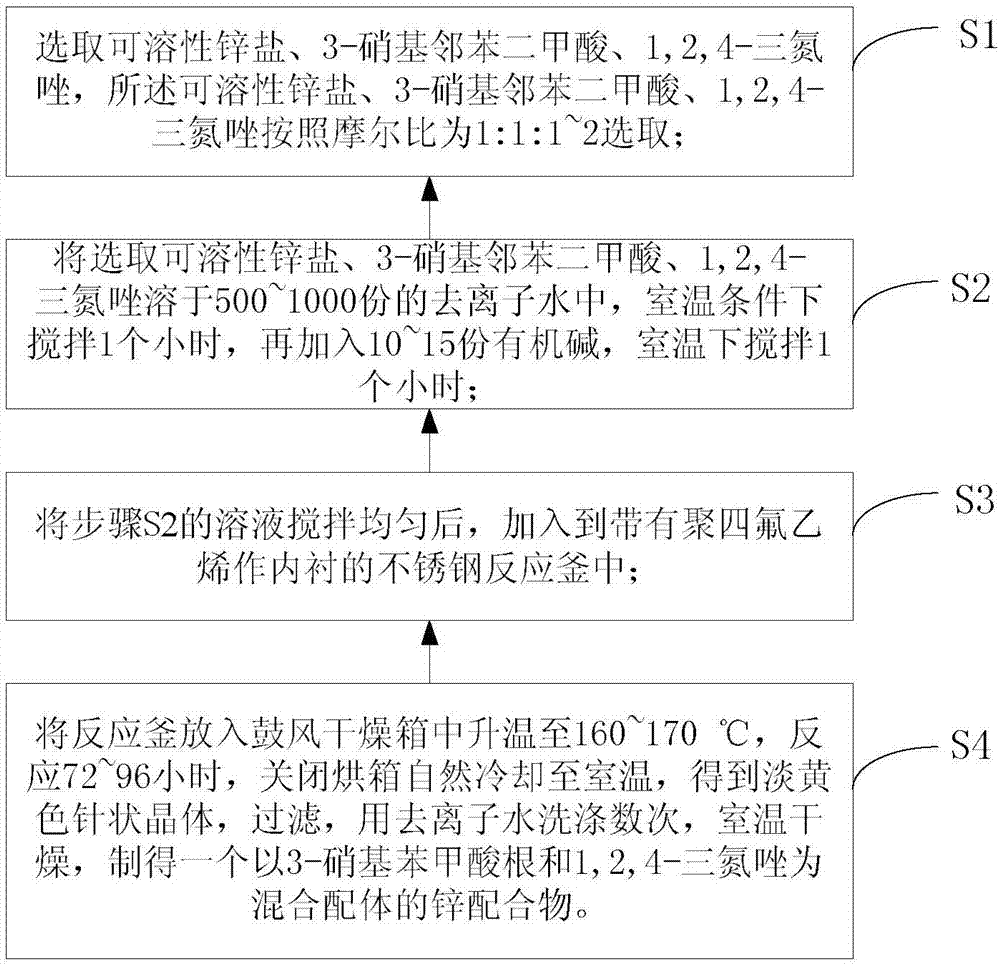
[0034] combine figure 1 , a method for preparing a two-dimensional zinc coordination polymer, comprising the following steps:
[0035] S1, choose soluble zinc salt, 3-nitrophthalic acid, 1,2,4-triazole, the soluble zinc salt, 3-nitrophthalic acid, 1,2,4-triazole Select according to the molar ratio of 1:1:1~2;
[0036] S2. Dissolve the selected soluble zinc salt, 3-nitrophthalic acid, and 1,2,4-triazole in 500-1000 parts of deionized water, stir at room temperature for 1 hour, and then add 10-15 Part of organic base, stirred at room temperature for 1 hour;
[0037] S3, after stirring the solution in step S2 evenly, add it into a stainless steel reaction kettle with polytetrafluoroethylene as the inner liner;
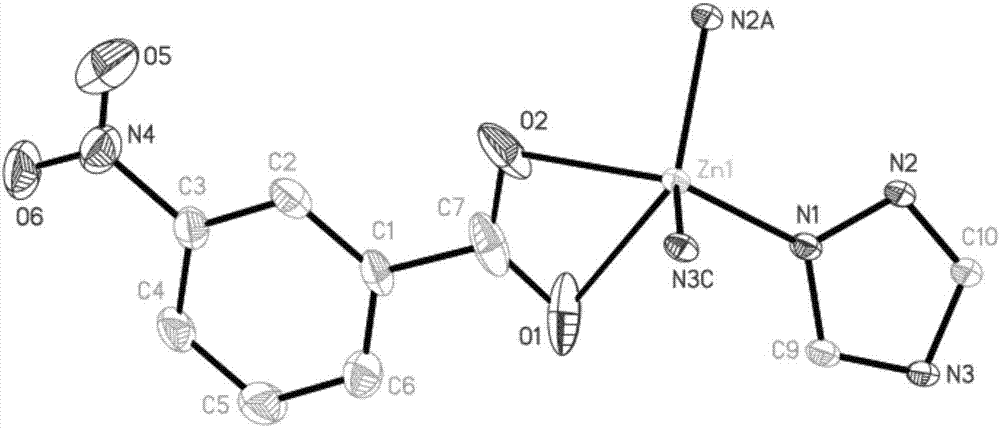
[0038]S4. Put the reaction kettle into a blast drying oven and heat up to 160-170°C, react for 72-96 hours, turn off the oven and cool to room temperature naturally to obtain light yellow needle-shaped crystals, filter, wash with deionized water several times, and brin...
Embodiment 1
[0040] A method for preparing a two-dimensional zinc coordination polymer, comprising the following steps:
[0041] S1, select zinc nitrate hexahydrate (0.1mmol), 3-nitrophthalic acid (0.1mmol) 1,2,4-triazole (0.2mmol), zinc nitrate hexahydrate, 3-nitrophthalic acid Formic acid and 1,2,4-triazole are selected according to the molar ratio of 1:1:2;
[0042] S2. Dissolve zinc nitrate hexahydrate, 3-nitrophthalic acid, and 1,2,4-triazole in 500 to 1,000 parts of deionized water, stir for 1 hour at room temperature, and then add triethyl Amine (1.0mmol), stirred at room temperature for 1 hour;
[0043] S3. After the solution in step S2 was stirred evenly, it was added to a 30 mL stainless steel reactor with polytetrafluoroethylene as a lining, and 10 mL of deionized water was added;
[0044] S4. Put the reaction kettle into a blast drying oven and heat up to 160°C, react for 72 hours, turn off the oven and cool to room temperature naturally to obtain light yellow needle-shaped c...
Embodiment 2
[0046] A method for preparing a two-dimensional zinc coordination polymer, comprising the following steps:
[0047] S1, choose zinc sulfate heptahydrate (0.1mmol), 3-nitrophthalic acid (0.1mmol) 1,2,4-triazole (0.2mmol), zinc sulfate heptahydrate, 3-nitrophthalate Formic acid and 1,2,4-triazole are selected according to the molar ratio of 1:1:1;
[0048] S2. Dissolve zinc sulfate heptahydrate, 3-nitrophthalic acid, and 1,2,4-triazole in 500 to 1,000 parts of deionized water, stir for 1 hour at room temperature, and then add triethyl Amine (1.5mmol), stirred at room temperature for 1 hour;
[0049] S3. After the solution in step S2 was stirred evenly, it was added to a 30 mL stainless steel reactor with polytetrafluoroethylene as a lining, and 10 mL of deionized water was added;
[0050] S4. Put the reaction kettle into a blast drying oven and heat up to 170°C, react for 96 hours, turn off the oven and cool to room temperature naturally to obtain light yellow needle-shaped cr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com