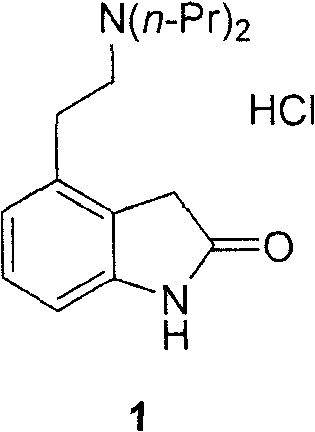
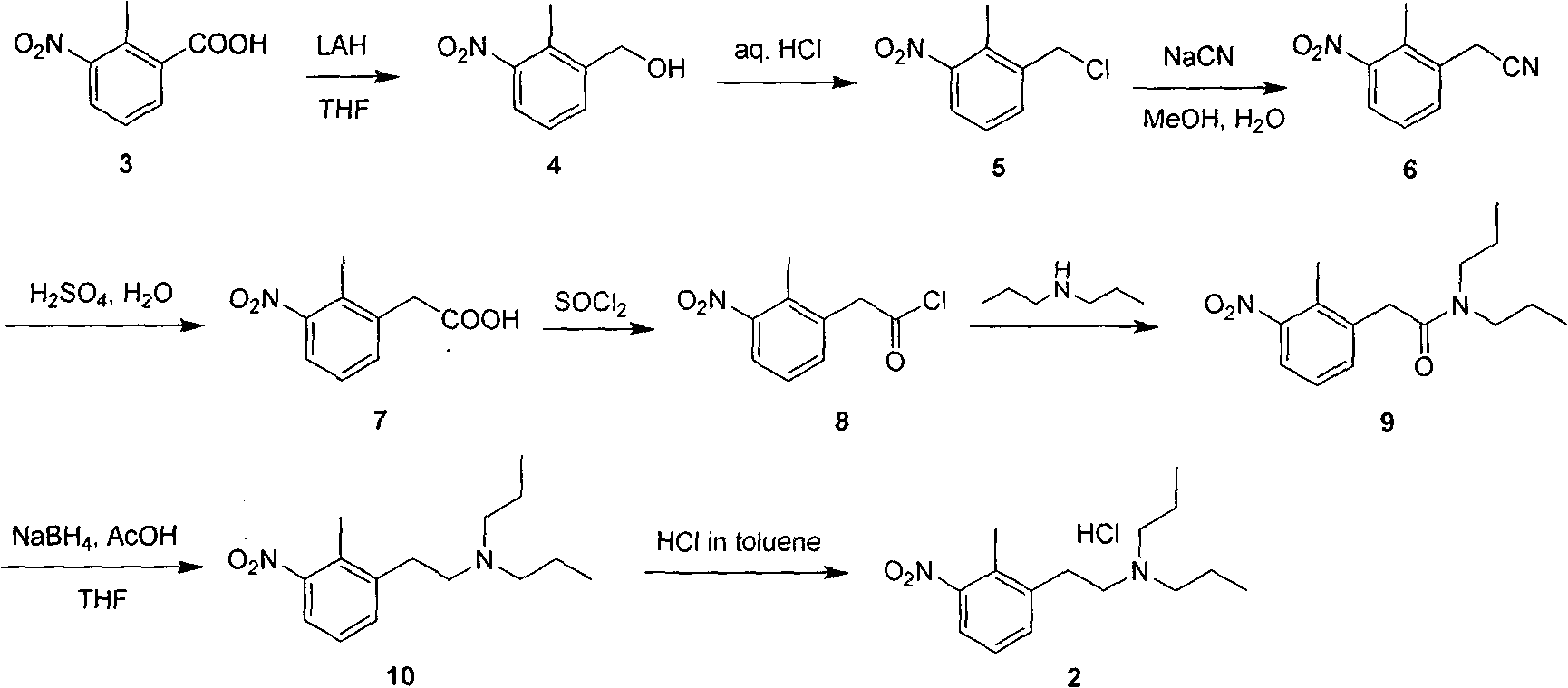
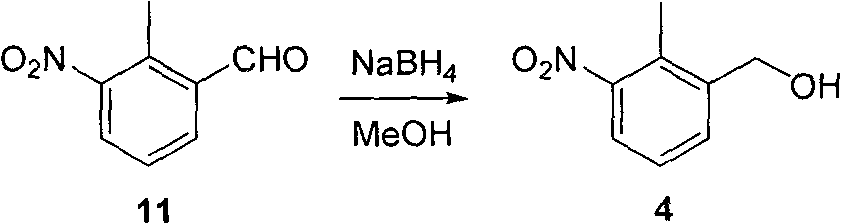Method for preparing N-(2-methyl-3-nitro)-N-propyl-1-propylamin hydrochloride
A technology of propylamine hydrochloride and nitrophenyl, which can be used in the preparation of amino compounds and organic chemistry from amines, and can solve the problems of long route, long production cycle, and many processes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of 3-(2-methyl-3-nitrophenyl)-glycidic acid methyl ester
[0031] 2-Methyl-3-nitrobenzaldehyde (50g, 0.3mol) and methyl chloroformate (39.5g, 0.36mol) were dissolved in benzene (500mL), the reaction solution was cooled to -5 degrees, and then kept below 0 degrees A methanol solution of sodium methoxide (19 g, 0.36 mol) was added dropwise. After the dropwise addition, tetrabutylammonium bromide (5 g, 0.015 mol) was added, and the mixture was kept at zero and stirred for 2 hours, and stirred overnight at room temperature. Water (200 mL) was added to the reaction solution, and the layers were separated. The organic phase was dried over anhydrous sodium sulfate and concentrated to obtain a yellow oil (42.4 g, yield 59%).
Embodiment 2
[0033] Synthesis of 2-methyl-3-nitrophenylacetaldehyde
[0034] 3-(2-Methyl-3-nitrophenyl)-glycidic acid methyl ester (30g, 0.13mol) was dissolved in 100mL of methanol, cooled to zero in an ice bath, NaOH (5.7g, 0.14mol) was added dropwise aqueous solution (100 mL), the reaction solution was slowly raised to room temperature, and stirred for 5 hours. Concentrate and distill off methanol, control the water layer below 10°C, adjust the pH to weak acidity with concentrated hydrochloric acid, add toluene (300mL), heat to reflux for 4 hours, separate the organic layer, wash with water, dry over anhydrous sodium sulfate, and concentrate to obtain a yellow oil , standing and cooling to obtain a yellow solid (15.2 g, yield 67%).
Embodiment 3
[0036] Synthesis of N-(2-methyl-3-nitro)-N-propyl-1-propanamine hydrochloride
[0037] 2-Methyl 3-nitrophenylacetaldehyde was dissolved in methanol (10g, 0.056mol), anhydrous magnesium sulfate (10g, 0.084mol) and di-n-propylamine (8.5g, 0.084mol) were added, and then cyano Sodium borohydride (3.5g, 0.056mol) in methanol (100mL) solution, the reaction solution was stirred overnight at room temperature, filtered, the filtrate was concentrated to dryness, the residue was dissolved in dichloromethane, washed with water, the aqueous layer was extracted twice with dichloromethane, The organic phases were combined and concentrated to give a light yellow oil, which was dissolved in toluene, and the toluene solution of HCL was slowly added dropwise to precipitate a white solid, which was cooled and filtered, and the obtained solid was recrystallized with 95% ethanol to obtain the product (11.6 g, yield 79%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com