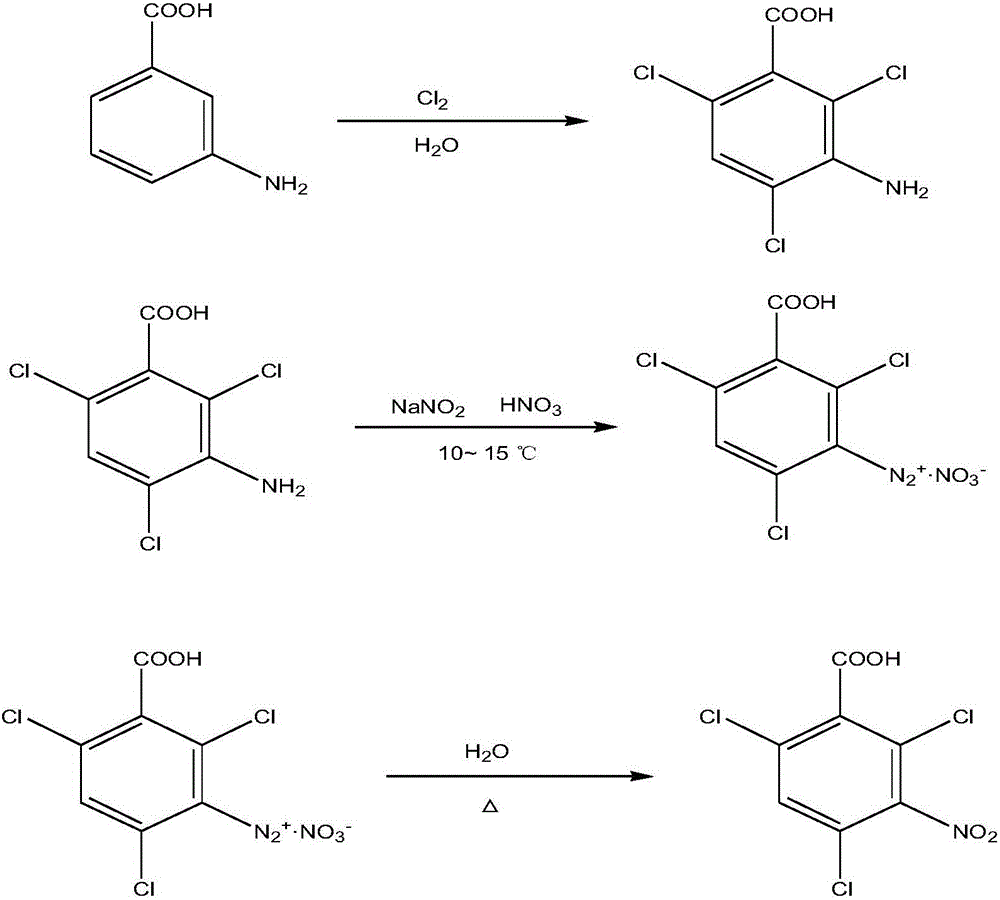
Synthetic method of 2,4,6-trichloro-3-nitrobenzoic acid
A technology of nitrobenzoic acid and synthesis method, which is applied in the field of compound synthesis, and can solve problems such as outdated technology, difficult management, and long process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0016] Add 10mL of m-aminobenzoic acid with a concentration of 0.1mol / L to a 250mL round bottom flask, then add 50mL of anhydrous ether, place it on a magnetic stirrer, and stir at a speed of 300r / min for 10min to fully dissolve it; keep the magnetic stirring speed While stirring, chlorine gas was introduced into the flask at a rate of 10mL / min, 10mL was ventilated, the shaking table was shaken for 30min, then 100mL of deionized water was added, stirring was continued for 10min, and the mixture was put into a Buchner funnel for extraction. Filter the filter residue; weigh 10 g of the filter residue obtained above and put it into a 250 mL flask, add 40 mL of sodium hydroxide solution with a concentration of 0.2 mol / L, put it in an ice-water bath, and stir it with a glass rod at 10°C for 10 minutes; after the stirring is completed 15mL of sodium nitrite solution with a mass concentration of 35% was added dropwise to the flask, and then 30mL of a concentrated nitric acid solution ...
example 2
[0018] Add 13mL of m-aminobenzoic acid with a concentration of 0.1mol / L to a 250mL round-bottom flask, then add 60mL of anhydrous ether, place it on a magnetic stirrer, and stir at a speed of 350r / min for 15min to fully dissolve it; keep the magnetic stirring speed While stirring, chlorine gas was introduced into the flask at a rate of 15mL / min, 15mL was ventilated, the shaking table was shaken for 35min, and then 105mL of deionized water was added, stirring was continued for 15min, and the mixture was put into a Buchner funnel for pumping. Filter the filter residue; weigh 15g of the filter residue obtained above and put it into a 250mL flask, add 50mL of sodium hydroxide solution with a concentration of 0.2mol / L, put it in an ice-water bath, and stir it with a glass rod at 13°C for 13min; after the stirring is completed 18mL of sodium nitrite solution with a mass concentration of 35% was added dropwise to the flask, and then 35mL of a concentrated nitric acid solution with a m...
example 3
[0020] Add 15mL of m-aminobenzoic acid with a concentration of 0.1mol / L to a 250mL round-bottom flask, then add 70mL of anhydrous ether, place it on a magnetic stirrer, and stir at a speed of 400r / min for 20min to fully dissolve it; keep the magnetic stirring speed While stirring, chlorine gas was introduced into the flask at a rate of 20mL / min, 20mL was ventilated, the shaking table was shaken for 40min, then 110mL of deionized water was added, stirring was continued for 20min, and the mixture was put into a Buchner funnel for pumping. Filter the filter residue; weigh 20g of the filter residue obtained above and put it into a 250mL flask, add 60mL of sodium hydroxide solution with a concentration of 0.2mol / L, put it in an ice-water bath, and stir it with a glass rod at 15°C for 20min; after the stirring is completed , add dropwise 20mL of sodium nitrite solution with a mass concentration of 35% into the flask, and then dropwise add 40mL of a concentrated nitric acid solution w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com