Non-cyanide gold plating bath and preparation method and application thereof
A technology of cyanide-free gold plating and gold-plating solution, which is applied in the field of gold-plating solution, and can solve problems such as poor stability of current efficiency and poor stability of cyanide-free gold-plating solution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
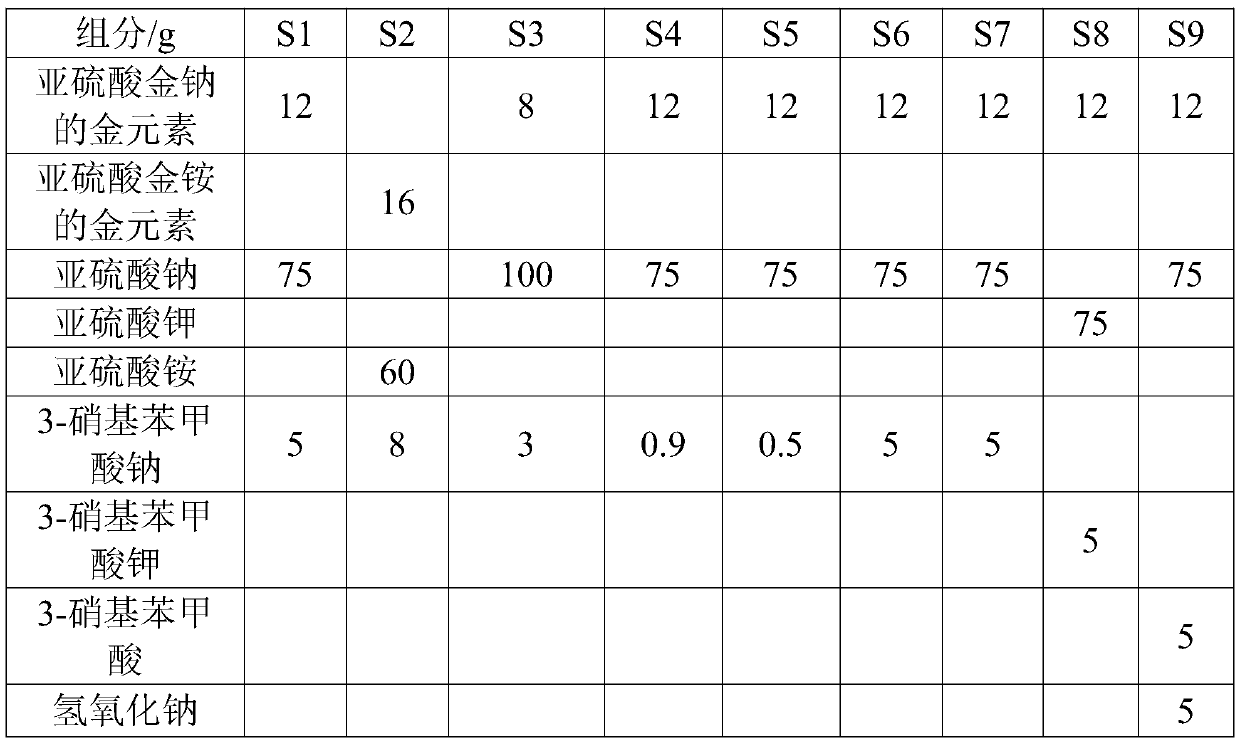
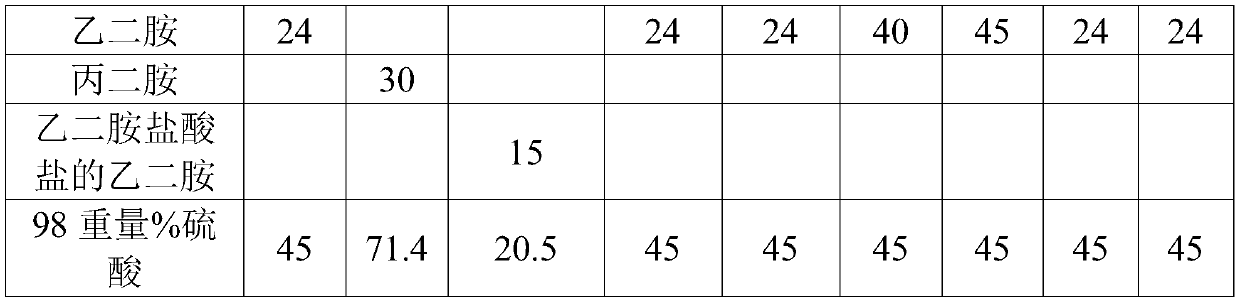
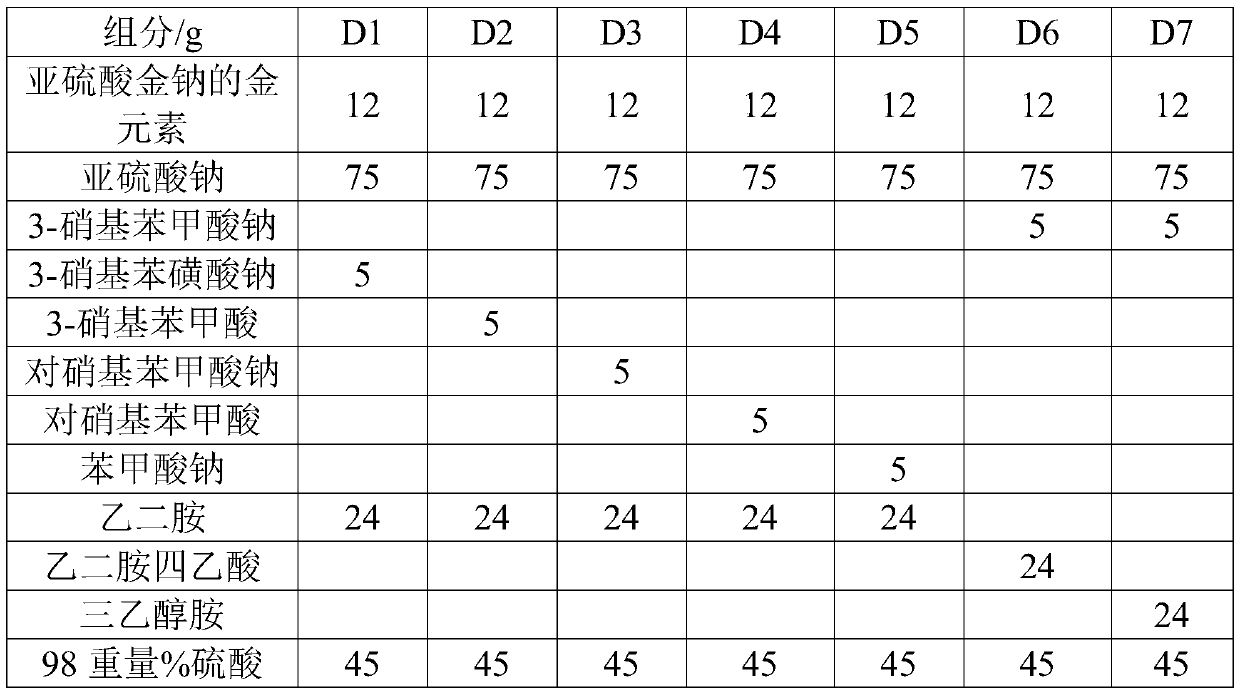
[0043] At 60°C, dissolve gold sodium sulfite, 75g sodium sulfite, 5g 3-nitrobenzoate, 24g ethylenediamine and 45g 98% by weight sulfuric acid in deionized water for 20min at 60°C. Prepare 1 L of cyanide-free gold-plating solution S1 with a pH value of 7.2, and use the cyanide-free gold-plating solution S1 to electroplate on a Hall cell test piece to form a coating with a thickness of 120 μm.
[0044] During the electroplating process, adjust the current density at 0.2-0.6A / dm 2 During the period, the current efficiency remained stable and greater than 98%; the surface of the product was smooth and uniform in color; and after 10 MTO productions, the cyanide-free gold plating solution S1 was still stable, and no turbidity or gold precipitation occurred.
Embodiment 2
[0046] At 55°C, gold ammonium sulfite of 16g, ammonium sulfite of 60g, sodium 3-nitrobenzoate of 8g, propylenediamine of 30g and sulfuric acid of 98% by weight of 71.4g were dissolved in Prepare 1 L of cyanide-free gold plating solution S2 with a pH value of 7 in deionized water at a constant temperature for 30 minutes, and use cyanide-free gold plating solution S2 to electroplate on the Hall cell test piece to form a coating with a thickness of 120 μm.
[0047] During the electroplating process, adjust the current density at 0.2-0.6A / dm 2 During the period, the current efficiency remained stable and was greater than 96%; the surface of the product was smooth and the color was uniform; and after 10 MTO productions, the cyanide-free gold plating solution S2 was still stable, and no turbidity or gold precipitation occurred.
Embodiment 3
[0049] At 65°C, ethylenediamine hydrochloride with a gold element content of 8g, 100g of sodium sulfite, 3g of 3-nitrobenzoic acid sodium, 15g of ethylenediamine hydrochloride and 20.5g of 98% by weight Sulfuric acid was dissolved in deionized water at a constant temperature for 15 minutes to prepare 1 L of cyanide-free gold plating solution S3 with a pH value of 7.5, and the cyanide-free gold plating solution S3 was used to electroplate on the Hall cell test piece to form a coating with a thickness of 120 μm.
[0050] During the electroplating process, adjust the current density at 0.2-0.6A / dm 2 During the period, the current efficiency remained stable and was greater than 96%; the surface of the product was smooth and the color was uniform; and after 10 MTO productions, the cyanide-free gold plating solution S3 was still stable, and no turbidity or gold precipitation occurred.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com