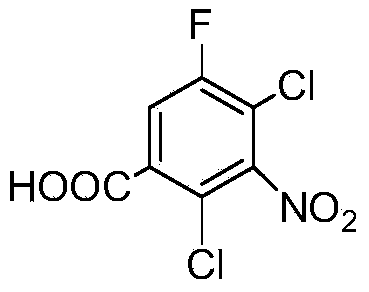
Preparation method of 2, 4-dichloro-5-fluoro-3-nitrobenzoic acid
A technology of nitrobenzoic acid and fluoroacetophenone, which is applied in the preparation of nitro compounds, organic chemistry, etc., can solve the problems of high cost and low product yield, achieve less three wastes, high product purity, and be conducive to industrialization Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
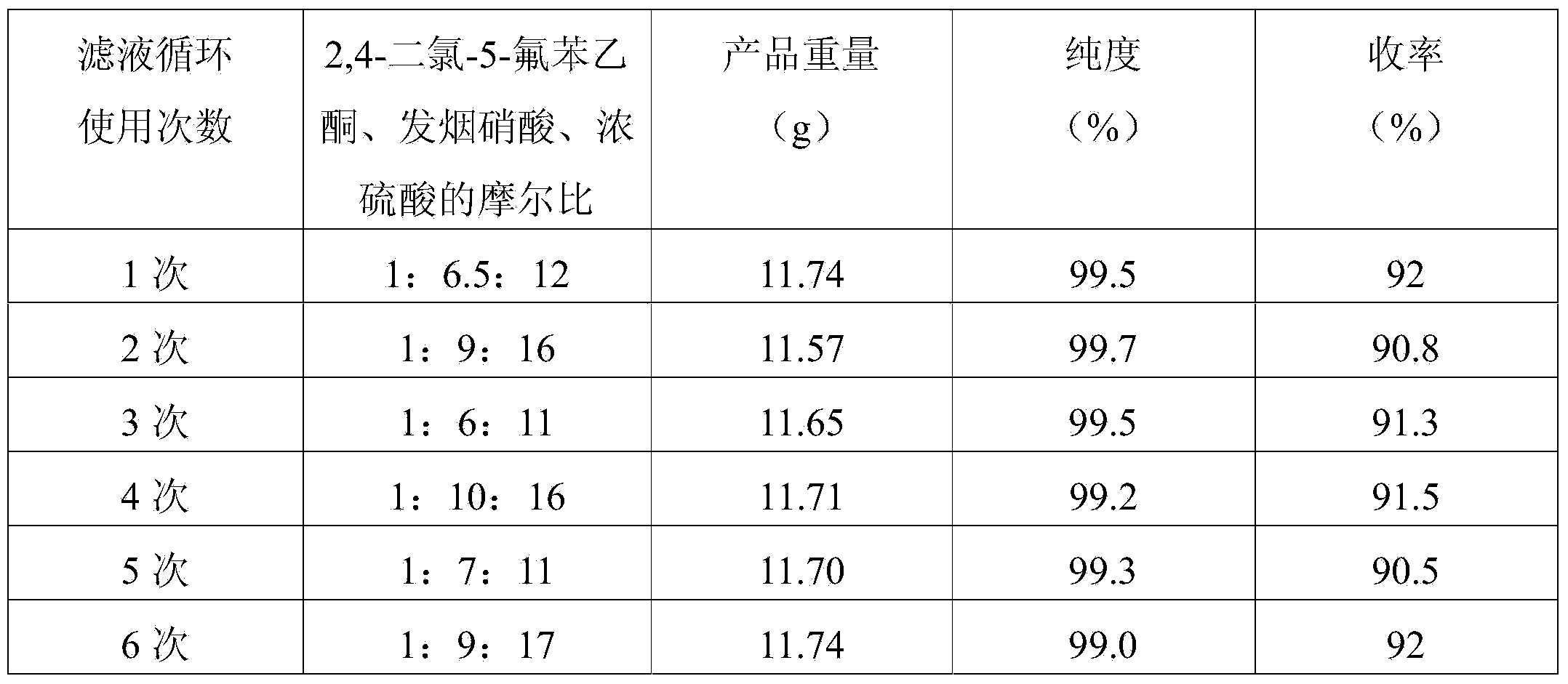
[0025] Add 80 grams (0.8mol) of concentrated sulfuric acid into a 250ml three-necked flask with a stirrer and a reflux pipe, utilize ice-cooled concentrated sulfuric acid, and add 28.93 grams (0.45 mol) fuming nitric acid, after the addition, the temperature of the mixed solution in the three-necked flask was raised to 103°C, and 10.46 grams (0.05mol) of 2,4-dichloro-5-fluoroacetophenone, which had been melted in advance, was added dropwise, The dropwise addition temperature was controlled at 103-110°C. After the dropwise addition was completed, it was kept at 103°C for 3 hours, cooled to 10°C and filtered, and the filtrate was recovered for later use. The filter cake was washed with water and then recrystallized with toluene to obtain 2,4-dichloro-5 - 11.61 g of fluoro-3-nitrobenzoic acid, with a purity of 99.3%, and a yield of 90.8% based on 2,4-dichloro-5-fluoroacetophenone.
Embodiment 2
[0027] Add 55 grams (0.55mol) of concentrated sulfuric acid into a 250ml three-necked flask with a stirrer and a reflux pipe, utilize ice-cooled concentrated sulfuric acid, and add 19.29 grams (0.3 mol) fuming nitric acid, after the addition, the temperature of the mixed solution in the three-necked flask was raised to 103°C, and 10.46 grams (0.05mol) of 2,4-dichloro-5-fluoroacetophenone, which had been melted in advance, was added dropwise, The dropwise addition temperature was controlled at 103-110°C. After the dropwise addition was completed, it was kept at 106°C for 3 hours, cooled to 10°C and filtered, and the filtrate was recovered for later use. The filter cake was washed with water and then recrystallized with toluene to obtain 2,4-dichloro-5 - Fluoro-3-nitrobenzoic acid 11.67 g, purity 99%, yield 91% based on 2,4-dichloro-5-fluoroacetophenone
Embodiment 3
[0029] Add 80 grams (0.8mol) of concentrated sulfuric acid into a 250ml three-necked flask with a stirrer and a reflux pipe, utilize ice-cooled concentrated sulfuric acid, and add 29.84 grams (0.45 mol) fuming nitric acid, after the addition, the temperature of the mixed solution in the three-necked flask was raised to 103°C, and 10.46 grams (0.05mol) of 2,4-dichloro-5-fluoroacetophenone, which had been melted in advance, was added dropwise, The dropwise addition temperature is controlled at 103-110°C. After the dropwise addition is completed, it is kept at 110°C for 3 hours, cooled to 10°C and filtered, and the filtrate is recovered for later use. After the filter cake is washed with water, it is recrystallized with toluene to obtain 2,4-dichloro-5- Fluoro-3-nitrobenzoic acid 11.46 g, purity 99.7%, yield 90% based on 2,4-dichloro-5-fluoroacetophenone
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com