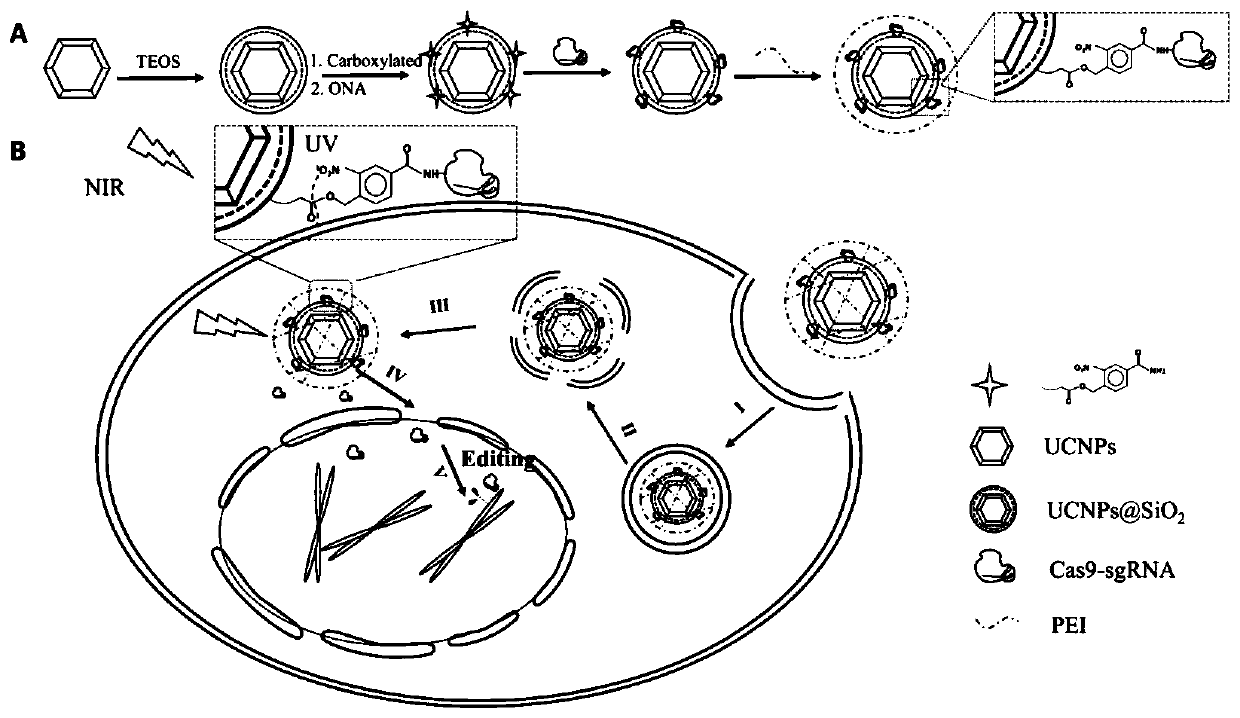
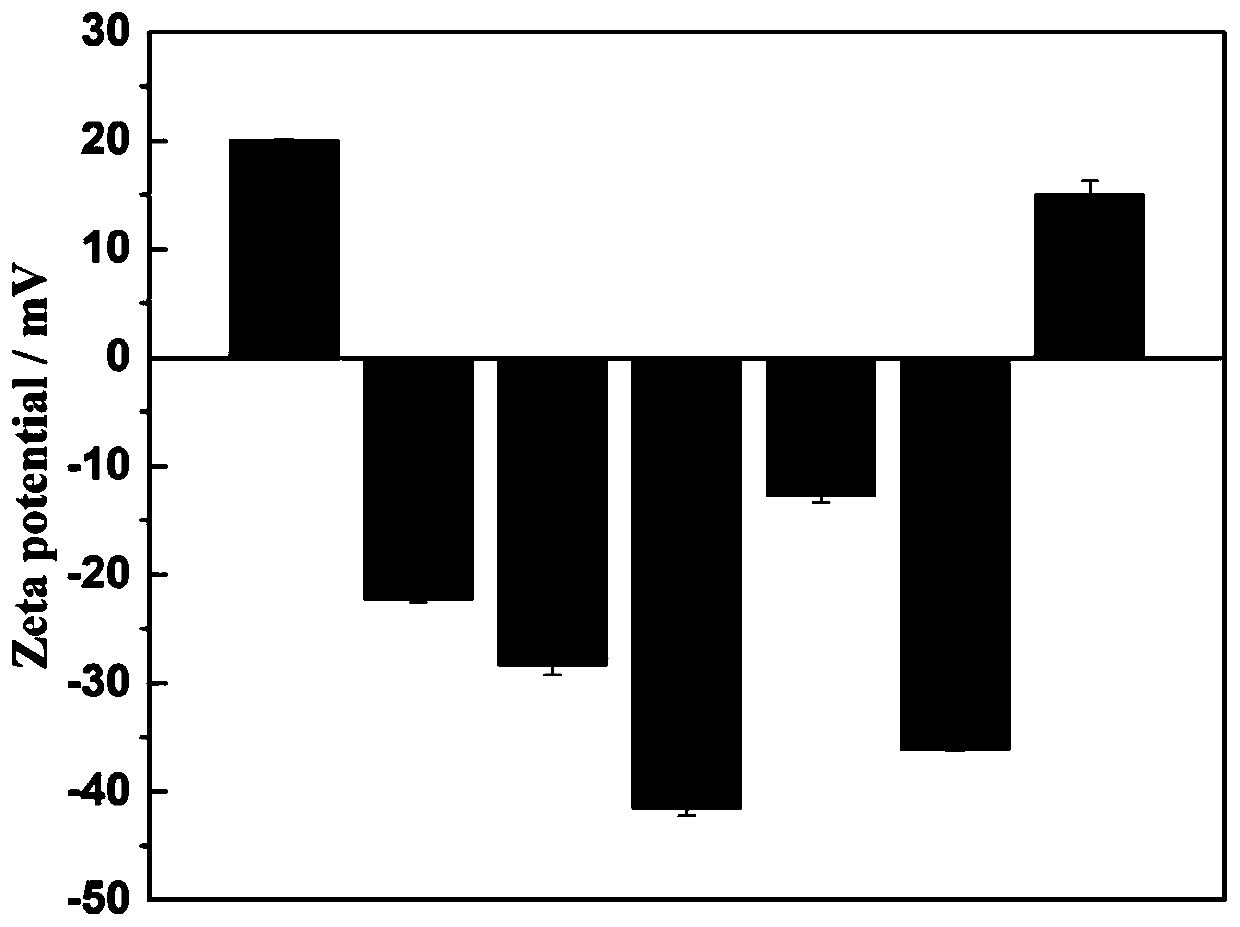
Near-infrared light controlled gene editing method
A technology of gene editing and infrared light, applied in the biological field, can solve problems such as poor penetration ability and canceration, and achieve the effect of improving targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] This embodiment provides the preparation method of UCNPs-Cas9@PEI:
[0036] UCNPs@SiO2 (100 mg) was carboxylated with 20 μL N-trimethoxysilylpropylethylenediaminetriacetic acid trisodium salt for 4.5 h. The carboxylated nanoparticles were then transferred to 10 mL (containing 100 μg ONA) of dry THF for esterification using dicyclohexylcarbodiimide and 4-dimethylaminopyridine as catalysts. After 12 hours, wash with THF 3 times, and then dissolve 20 μg of dicyclohexylcarbodiimide in THF to further activate the carboxyl group. After 8 hours, the particles were collected and transferred into PBS. At the same time, mix Cas9 protein and sgRNA (1:2) in PBS for 5 minutes to form a CRISPR / Cas9 system. Then, the CRISPR / Cas9 system was mixed with carboxylated nanoparticles-ONA (i.e. the aforementioned ligation product of carboxylated nanoparticles and ONA), and o C incubated overnight. After centrifugation, PEI was coated on UCNPS-Cas9 at a weight ratio of 5:1 (PEI:sgRNA), and...
Embodiment 2
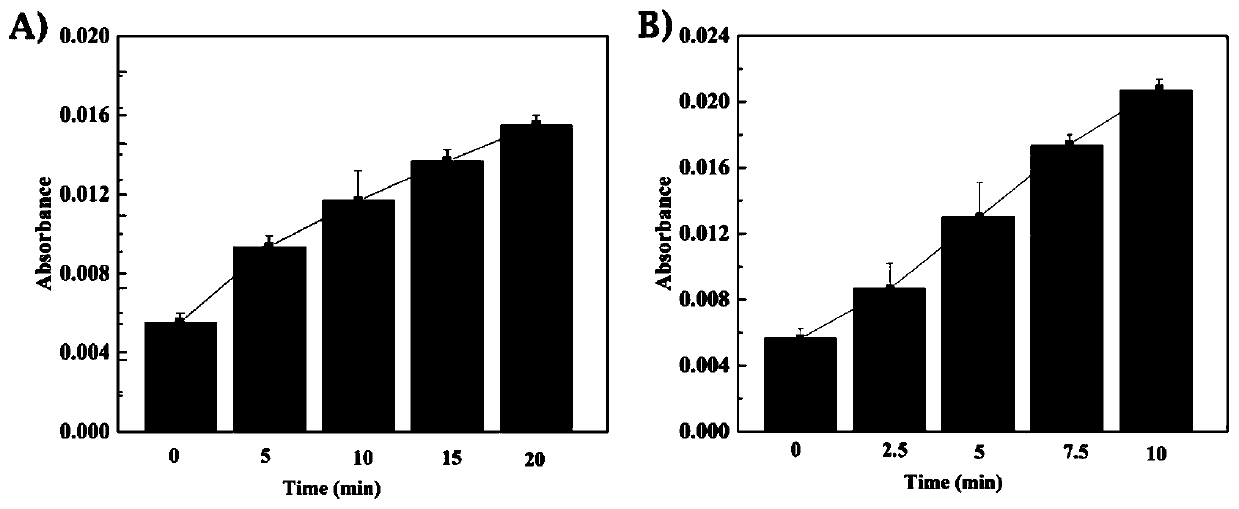
[0038] This embodiment verifies the near-infrared response of the system, the method is as follows:
[0039] Using a collimator, fix the light intensity of the 980 nm near-infrared laser at 2 W / cm 2 . Put the UCNPs-Cas9 solution under the light intensity, centrifuge every 5 minutes to obtain the supernatant, and measure the UV absorption peak at 280 nm (common protein absorption peak), the results are as follows image 3 As shown in A, the absorption peak increases with time, which means that the protein is detached from the particle. In addition, in order to verify the role of upconversion nanoparticles in converting infrared light into ultraviolet light, the UCNPs-Cas9 solution can be exposed to ultraviolet light, centrifuged every 2.5 minutes to obtain the supernatant to measure the 280 nm absorption peak, and similar results were obtained Such as image 3 b.
Embodiment 3
[0041] The nanocomplex of test embodiment 1 (sgRNA targeting EGFP Genes whose targeting sequences are:
[0042] 5'-GGGCGAGGAGCTGTTCACCG-3' (SEQ ID NO.1)) cytotoxicity, the method is as follows:
[0043] Select lung cancer cells (A549) and irradiate them under different intensities of infrared light for 10 minutes. According to the results obtained by the CCK-8 detection method, 0.5~2.5 W / cm 2 Infrared light does not have much effect on cell activity ( Figure 4 A). Subsequently, at 2.0W / cm 2 Under the infrared light intensity, different concentrations of UCNPs-Cas9@PEI complexes were selected to incubate with cells, and it was found that the toxicity of nanoparticles to cells increased as the concentration increased ( Figure 4 B). Finally, the effect of different light durations on cell activity was investigated. In order to reduce the toxicity of the UCNPs-Cas9@PEI complex itself and infrared light conditions on cells, in subsequent cell experiments, 2.0 W / cm 2 The inf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com