New preparation method of 2-methyl-3-nitrobenzoic acid
A technology of nitrobenzoic acid and nitro-o-xylene, which is applied in the new field of preparation of 2-methyl-3-nitrobenzoic acid, can solve the problems of high pollution, high risk, and easy explosion, and achieve the cost of raw materials Low, less pollution, less dangerous effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
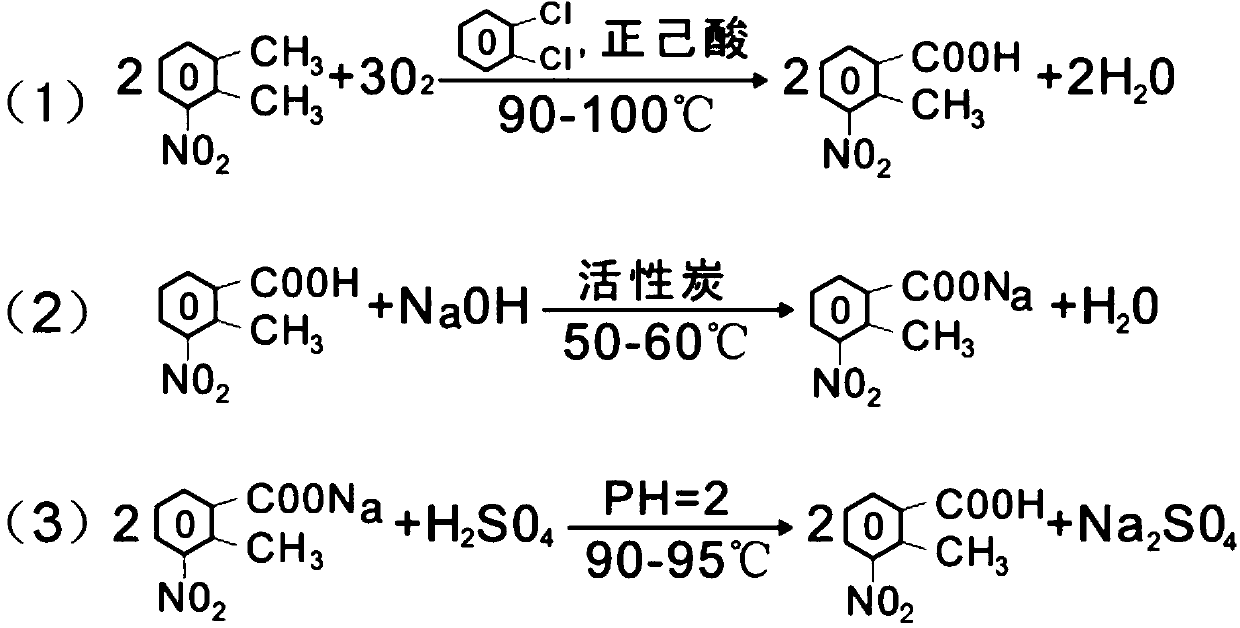
[0017] Example 1: Put 200g of 3-nitro-o-xylene, 700g of o-dichlorobenzene, 200g of n-hexanoic acid, 12g of cobalt acetate, 12g of manganese acetate, and 6g of tetrabromoethane into a 1000ml four-necked flask with a reflux condenser and a water separator. , feed oxygen to maintain 1.2L / min, raise the temperature to 90°C, keep the temperature at 90-100°C for 18 hours, and separate out about 23g of water, and control 3-nitro-o-xylene ≤ 1% in the sampling, which is the end point of the reaction, if the raw material More, continue to pass through the oxygen reaction until the end of the reaction; cool and filter to get 235g of crude product (containing solvent); the mother liquor is used as the next batch of solvent and then add a certain amount of catalyst, and add 5% of the original amount of catalyst, throw 3-Nitrate The base o-xylene reacts with oxygen again, and the result is the same as the original batch.
[0018] Put 235g of crude product, 1400ml of water, and 45g of causti...
example 2
[0019] Example 2: Put 200g of 3-nitro-o-xylene, 700g of o-dichlorobenzene, 300g of n-hexanoic acid, 4g of cobalt acetate, 2g of manganese acetate, and 4g of tetrabromoethane into a 1000ml four-necked flask with reflux condenser and water separator , keep the air at 3.5L / min, raise the temperature to 90°C, and keep the reaction at 90-100°C for 18 hours, and about 23g of water will be separated out. The control of 3-nitro-o-xylene in the sampling is ≤1%, which is the end point of the reaction. If the raw material More, continue the reaction with oxygen until the reaction reaches the end; cool and filter to obtain 240g of crude product (containing solvent).
[0020] Put 240g crude product into 2000ml four-necked bottle, 1400ml water, 29g purity is 99% caustic soda, heat up to 50-60 ℃, react for 30 minutes, place separatory funnel, separate oil 35g (use as next batch of reaction solvent) , return the water to the 2000ml four-neck bottle, add 6g of activated carbon, stir and decolo...
example 3
[0021] Example 3: Put 200g 3-nitro-o-xylene, 200g o-dichlorobenzene, 200g n-hexanoic acid, 10g cobalt acetate, 14g manganese acetate, 16g tetrabromoethane into a 1000ml four-necked bottle with reflux condenser and water separator , feed oxygen to maintain 1.0L / min, raise the temperature to 90°C, keep the temperature at 90-100°C for 18 hours, and separate out about 23g of water, and control 3-nitro-o-xylene ≤ 1% in sampling, which is the end point of the reaction, if the raw material More, continue to feed oxygen reaction, until the reaction reaches the end; cooling and filtering to get crude product 230g (containing solvent);
[0022] Drop into 230g crude product in the 2000ml four-necked bottle, 1400ml water, 73.7g purity is 99% caustic soda, is warming up to 50-60 ℃, reacts 30 minutes, is placed in separating funnel, separates oil 25g (uses as next batch reaction solvent) ), return the water to the 2000ml four-neck bottle, add 6g of activated carbon, stir and decolorize at 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com