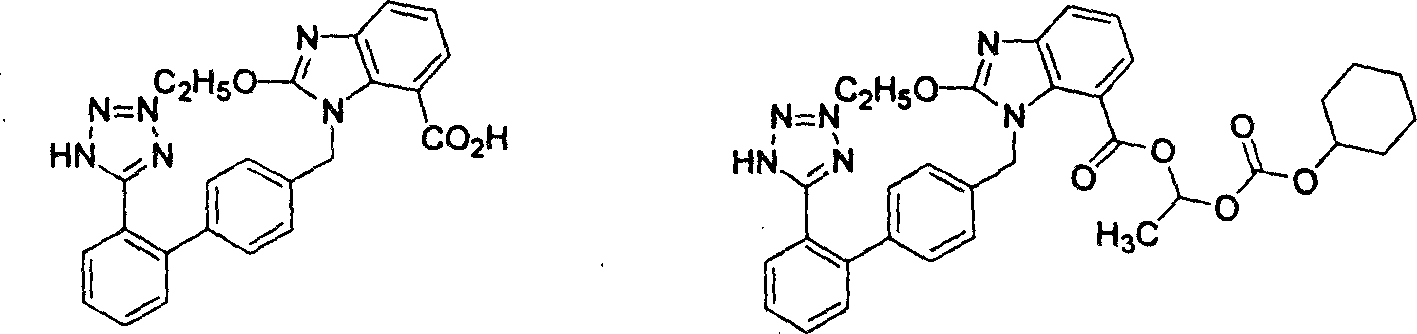
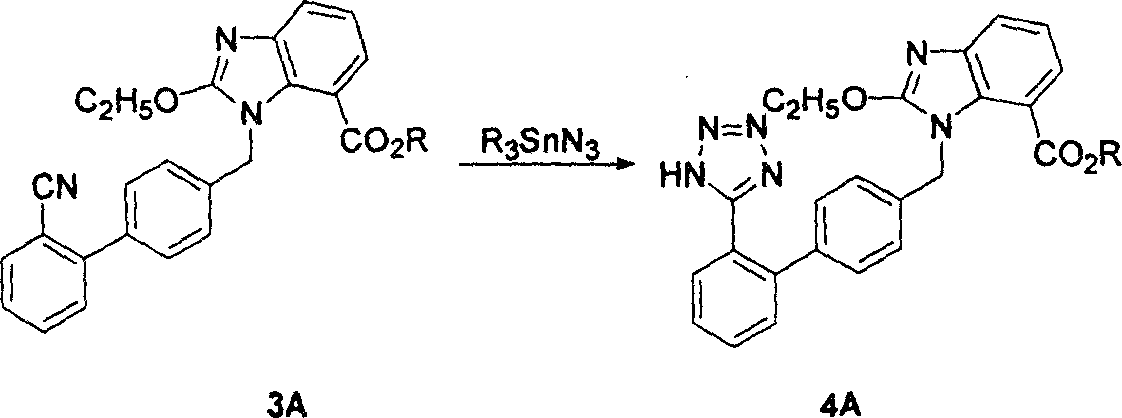
Method for preparing candestartan
A candesartan and reaction technology, which is applied to a preparation field of candesartan, can solve the problems of harsh operating conditions, long reaction time, pollution of poisonous reagents and the like, and achieves the effect of simple operation process and easy industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
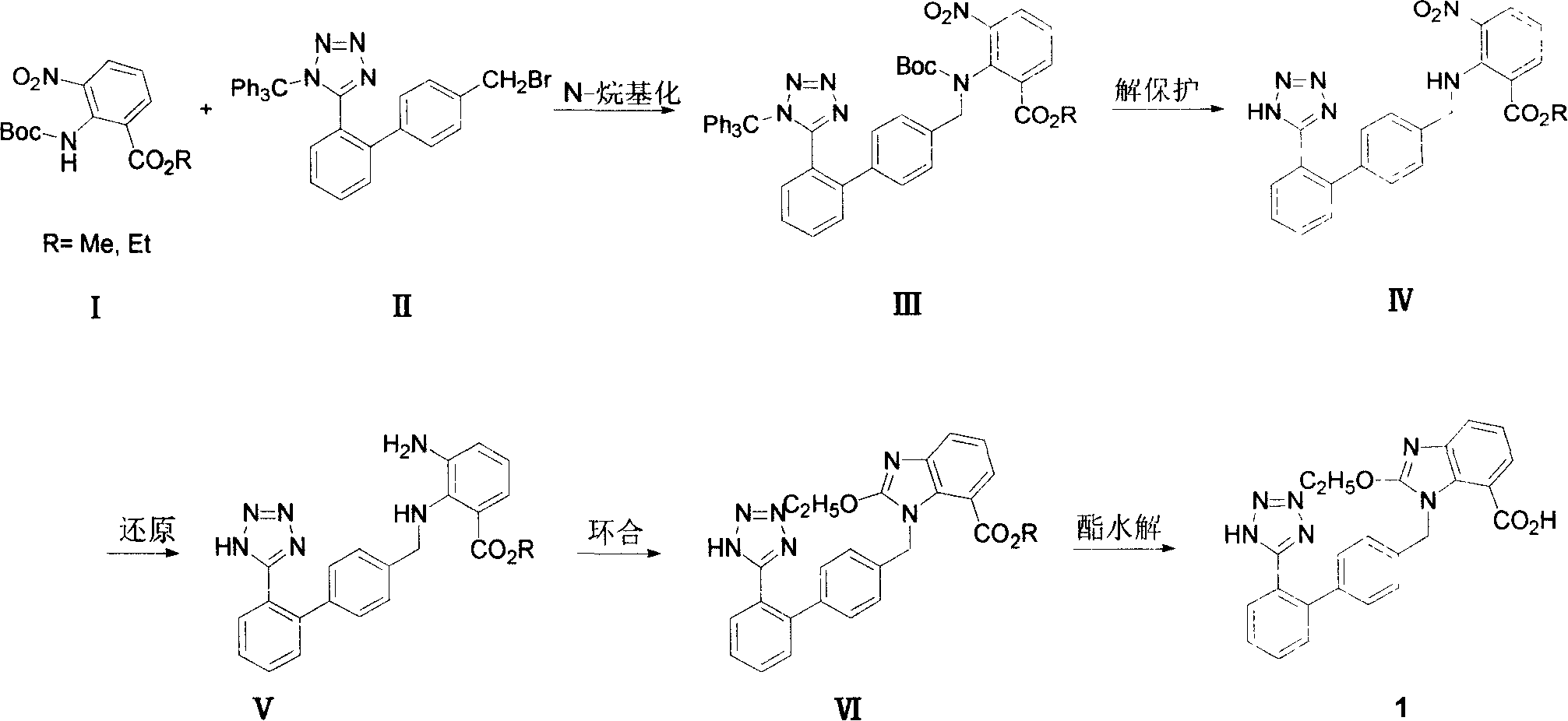
[0056] Example 1.2-[N-(tert-butoxycarbonyl)-N-[[(2'-(N'-trityl)-tetrazol-5-yl)[1,1'-biphenyl]- Preparation of ethyl 4-yl]methyl]amino]-3-nitrobenzoate (III, R=ethyl)
[0057] In a 1000mL four-neck flask equipped with a thermometer, a reflux condenser, a drying tube, and a mechanical stirrer, add 51.4g of ethyl 2-tert-butoxycarbonylamino-3-nitrobenzoate (purity 92%, 0.15mol) and Acetonitrile 500ml, add potassium carbonate 32g (0.23mol) under stirring. After the mixture was stirred for 30 minutes, 89 g (content 95%, 0.15 mol) of N-(triphenylmethyl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium (II) was added, The reaction was stirred at reflux for 14 hours. Cool and filter, concentrate under reduced pressure and recover acetonitrile. The residue was dissolved in dichloromethane, washed with water, the organic phase was dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a pale yellow oil. The oil was Intermediate III, weighing 131 g. This pro...
Embodiment 22
[0058] Example 2.2-[N-[(2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl)methyl]amino]-3-nitrobenzoic acid ethyl Preparation of Esters (IV, R=Ethyl)
[0059] In a 1000mL four-neck flask equipped with a thermometer, reflux condenser, drying tube, and mechanical stirring, the 2-{N-(tert-butoxycarbonyl)-N-[(2'-(N'- Trityl)-tetrazolium-5-yl)biphenyl-4-yl]amino}-3-nitrobenzoic acid ethyl ester crude product was dissolved in the mixed solvent of 100ml ethyl acetate and 500ml methanol, heated to reflux for 24 hours, concentrated. The residue was crystallized with dichloromethane and petroleum ether, filtered, and the filter cake was washed with a little petroleum ether, sucked dry, and dried in vacuo to obtain 54.4 g of a light yellow solid, which was 2-[N-[(2'-(1H-tetraazole Azol-5-yl)biphenyl-4-yl)amino]-3-nitrobenzoic acid ethyl ester. Based on ethyl 2-tert-butoxycarbonylamino-3-nitrobenzoate, the two-step total yield was 81.5%. The resulting solid was recrystallized from dichloromet...
Embodiment 32
[0060] Example 3.2-[N-[(2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl)methyl]amino]-3-nitrobenzoic acid methyl Preparation of Esters (IV, R = Methyl)
[0061] In a 1000mL four-necked flask equipped with a thermometer, a reflux condenser, a drying tube, and a mechanical stirrer, add 47.7 g of methyl 2-tert-butoxycarbonylamino-3-nitrobenzoate (purity 93%, 0.15 mol) and Acetonitrile 500ml, add potassium carbonate 32g (0.23mol) under stirring. After the mixture was stirred for 30 minutes, 89 g (content 95%, 0.15 mol) of N-(triphenylmethyl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium (II) was added, The reaction was stirred at reflux for 14 hours. Cool and filter, concentrate under reduced pressure and recover acetonitrile. Add 150ml of ethyl acetate to the residue to dissolve, wash with water, dry the organic layer over anhydrous sodium sulfate, and filter. Add 600ml of methanol to the filtrate, heat to reflux for 24 hours, and concentrate. The residue was crystallized with dichlo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com