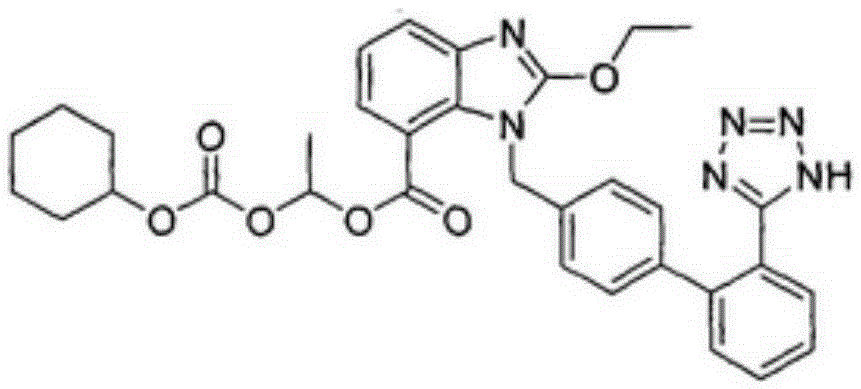
Preparation method of candesartan cilexetil
A technology of candesartan cilexetil and tetraethyl orthocarbonate, which is applied in the field of preparation of candesartan cilexetil, can solve problems such as difficulties in the industrial production of candesartan cilexetil, achieve high industrialization development prospects, simple process steps, The effect of easy purification of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
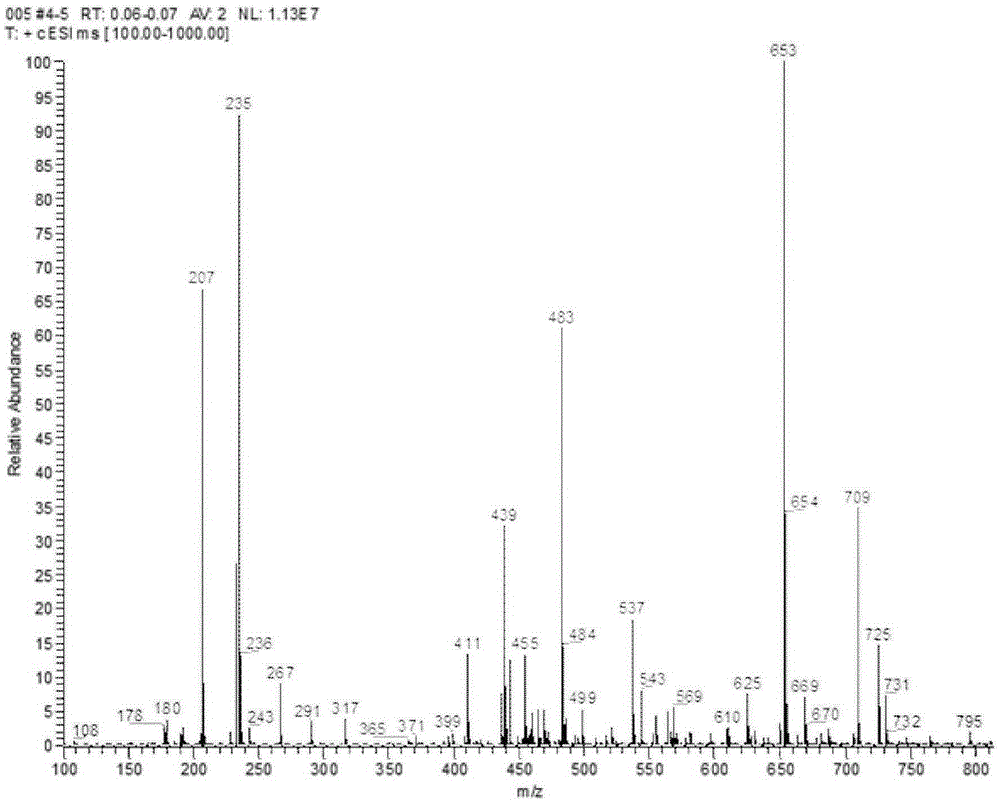
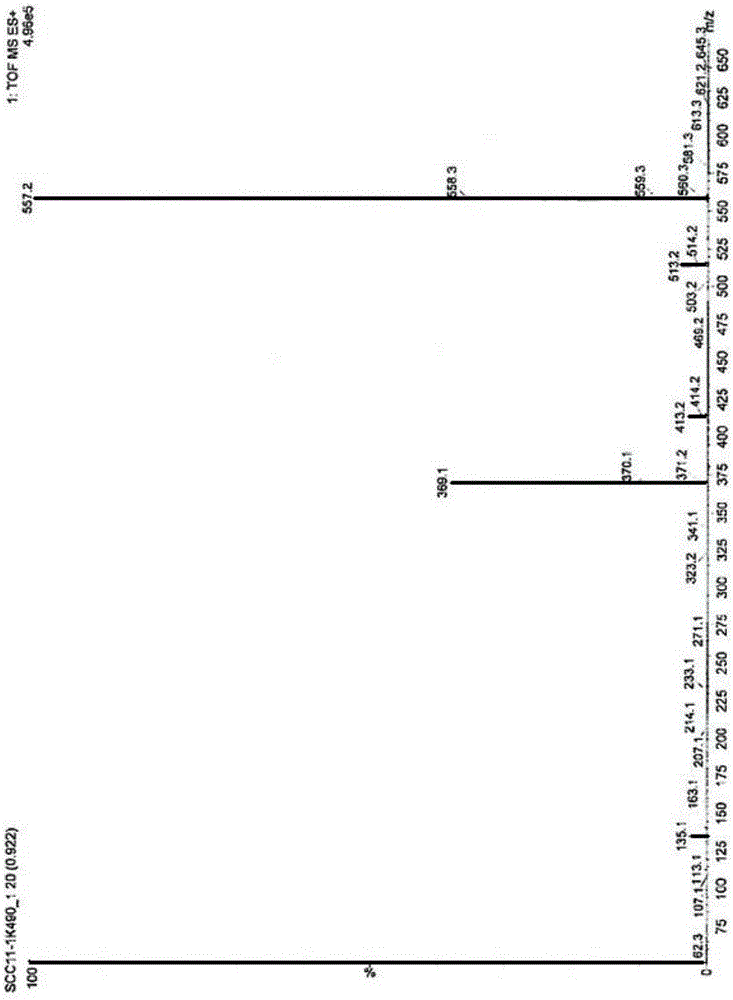
Image
Examples
Embodiment 1
[0028] The preparation method of the candesartan cilexetil of the present embodiment comprises the following steps:
[0029] (1) Alcoholyzing compound IV to obtain compound III: in a 1000mL four-neck flask equipped with a thermometer and mechanical stirring, add 0.1 moL of compound IV, namely 2-(tert-butoxycarbonyl ((2-(1-triphenyl Methyl-1H-tetrazol-5-yl)biphenyl-4-yl)methyl)amino)-3-nitrobenzoic acid-1-(((cyclohexyloxy)carbonyl)oxy)ethyl ester and 250mL methanol, reacted at 40-45°C for 3 hours, TCL detected that the reaction was complete, then lowered to room temperature, stirred at room temperature for 6-8 hours, filtered, and the filter cake was washed with 100mL glacial methyl tert-butyl ether, sucked dry, and vacuumed Dry to obtain 61.7 g of light yellow solid, which is compound III, 2-(tert-butoxycarbonyl((2-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl)amino )-1-(((cyclohexyloxy)carbonyl)oxy)ethyl ester of 3-nitrobenzoate, the yield is 90%, and the melting point is 105~106°C...
Embodiment 2
[0033] (1) Alcoholyze compound IV to obtain compound III: in a 1000mL four-neck flask equipped with a thermometer and mechanical stirring, add 0.1moL of 2-(tert-butoxycarbonyl ((2-(1-trityl- 1H-tetrazol-5-yl)biphenyl-4-yl)methyl)amino)-3-nitrobenzoic acid-1-(((cyclohexyloxy)carbonyl)oxy)ethyl ester, 50mL ethanol , 300mL ethyl acetate, add 1mL concentrated hydrochloric acid, stir at room temperature for 6-8 hours, after TCl detects that the reaction is complete, add 200mL of water, stir for 10 minutes, let stand to separate layers, concentrate the organic layer under reduced pressure to 100mL, cool to 0~ Stir at 5°C for 4-6 hours, filter, drain, and vacuum-dry to obtain 58.3 g of a light yellow solid, which is compound III, namely 2-(tert-butoxycarbonyl ((2-(1H-tetrazole -5-yl)biphenyl-4-yl)methyl)amino)-3-nitrobenzoic acid-1-(((cyclohexyloxy)carbonyl)oxy)ethyl ester, the yield was 85%.
[0034](2) Add 68.6 g of 2-(tert-butoxycarbonyl ((2-(1H-tetrazol-5-yl)biphenyl-4-yl)methan...
Embodiment 3
[0037] (1) Alcoholyze compound IV to obtain compound III: in a 1000mL four-neck flask equipped with a thermometer and mechanical stirring, add 0.1moL of 2-(tert-butoxycarbonyl ((2-(1-trityl- 1H-tetrazol-5-yl)biphenyl-4-yl)methyl)amino)-3-nitrobenzoic acid-1-(((cyclohexyloxy)carbonyl)oxy)ethyl ester, 50mL ethanol , 300mL ethyl acetate, add 1mL concentrated hydrochloric acid, stir at room temperature for 6-8 hours, after TCl detects that the reaction is complete, add 200mL of water, stir for 10 minutes, let stand to separate layers, concentrate the organic layer under reduced pressure to 100mL, cool to 0~ Stir at 5°C for 4-6 hours, filter, drain, and vacuum-dry to obtain 58.3 g of a light yellow solid, which is compound III, namely 2-(tert-butoxycarbonyl ((2-(1H-tetrazole -5-yl)biphenyl-4-yl)methyl)amino)-3-nitrobenzoic acid-1-(((cyclohexyloxy)carbonyl)oxy)ethyl ester, yield 85%;
[0038] (2) Add 2-(tert-butoxycarbonyl((2-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl)amino to a 1000mL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com