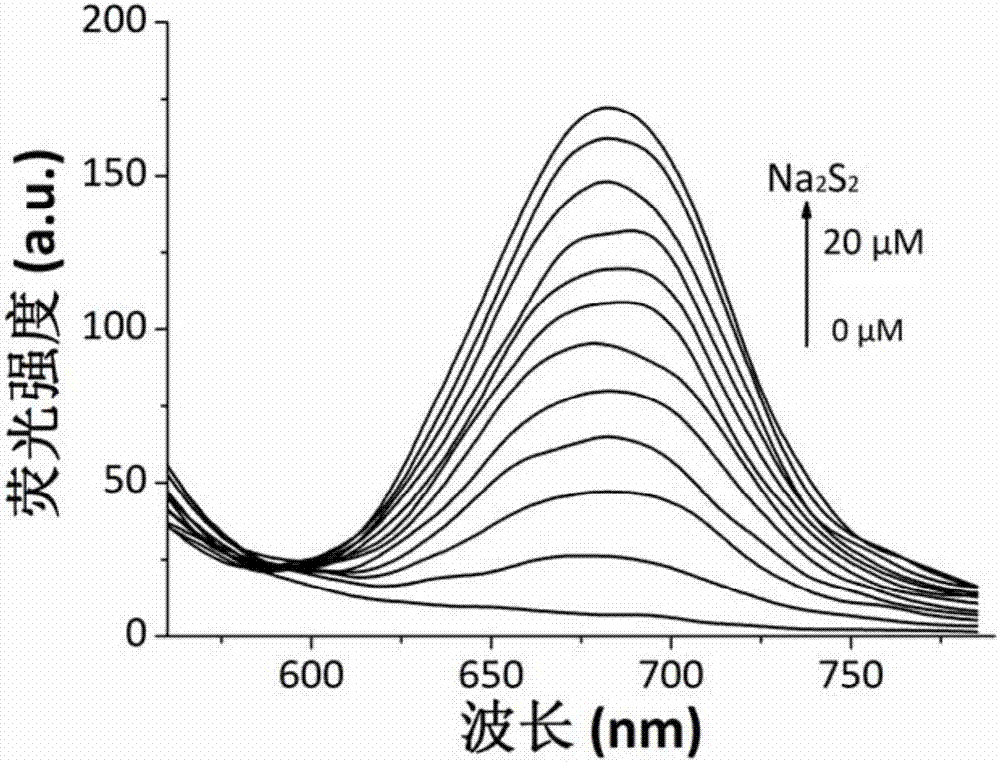
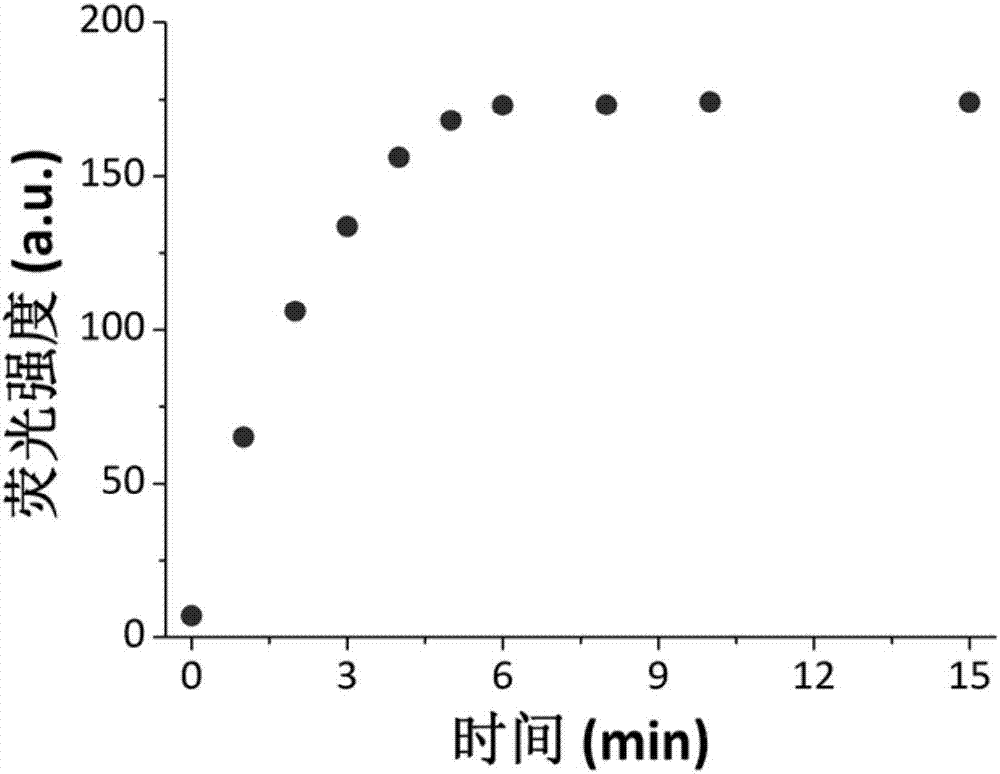
Synthesis and application of near infrared fluorescence probe for detecting hydrogen polysulfide
A fluorescent probe, hydrogen polysulfide technology, applied in the field of synthesis of near-infrared fluorescent probes, can solve problems such as poor selectivity, difficult popularization and application, and complicated measurement operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Preparation of compound III:
[0017] Under nitrogen protection, accurately weigh pyranonitrile compound II (500mg, 2.27mmol), p-hydroxybenzaldehyde (335g, 2.72mmol), dissolve in 20mL of acetonitrile, then add 1 drop of piperidine dropwise to the mixed solution, and reflux The reaction was stirred for 6 hours. TLC detected that the reaction was complete, the reaction was stopped, the solvent was distilled off under reduced pressure, and separated and purified by column chromatography to obtain a red product (480 mg, yield 67.5%). 1 H NMR (400MHz, DMSO-d 6 )δ10.29(s, 1H), 8.70(d, J=8.0Hz, 1H), 7.91(t, J=7.8Hz, 1H), 7.78(d, J=8.0Hz, 1H), 7.70-7.54( m,4H),7.23(d,J=16.0Hz,1H),6.95(s,1H),6.85(d,J=8.5Hz,2H). 13 C NMR (100MHz, DMSO-d 6 )δ160.0, 158.9, 152.8, 152.0, 139.3, 135.2, 130.3, 126.1, 126.0, 124.6, 118.9, 117.4, 117.1, 116.0, 115.9, 105.7, 59.1. For the synthetic steps of compound II, see RSC Adv., 2014, 4 ,46561-46567.
Embodiment 2
[0019] Preparation of compound I:
[0020] Under nitrogen protection, accurately weigh hydroxypyranonitrile compound II (300mg, 0.95mmol), 2-fluoro-5-nitrobenzoic acid (209mg, 1.15mmol), 1-ethyl-(3-dimethylamino Propyl)carbodiimide hydrochloride (363mg, 1.90mmol), 4-dimethylaminopyridine (12mg, 0.95mmol) were dissolved in 20mL of dichloromethane, and stirred at room temperature for 8 hours. TLC detected that the reaction was complete, the reaction was stopped, washed with saturated sodium chloride solution, the solvent was distilled off under reduced pressure, and separated and purified by column chromatography to obtain a light yellow product (180 mg, yield 39.6%). 1 H NMR (400MHz, DMSO-d 6 )δ8.70(d,J=12.0Hz,1H),8.63-8.60(m,1H),8.52-8.48(m,1H),7.90(t,J=8.0Hz,1H),7.76(d,J =8.0Hz, 2H), 7.66-7.57(m, 4H), 7.25(d, J=16.0Hz, 1H), 6.92(s, 1H), 6.85(d, J=8.0Hz, 2H). 13 C NMR (100MHz, DMSO-d 6 )δ166.2, 163.8, 160.5, 159.3, 153.2, 152.5, 144.1, 139.7, 135.7, 132.6, 130.8, 130.4, 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com