Electroreduction preparation method of anticancer drug gefitinib and analogue intermediate thereof
A technology of electrodes and derivatives, applied in the direction of electrolysis process, electrolysis components, electrolysis organic production, etc., can solve the problems of large environmental pollution, environmental pollution, expensive catalyst palladium, etc., and achieve the effect of simplifying the process and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
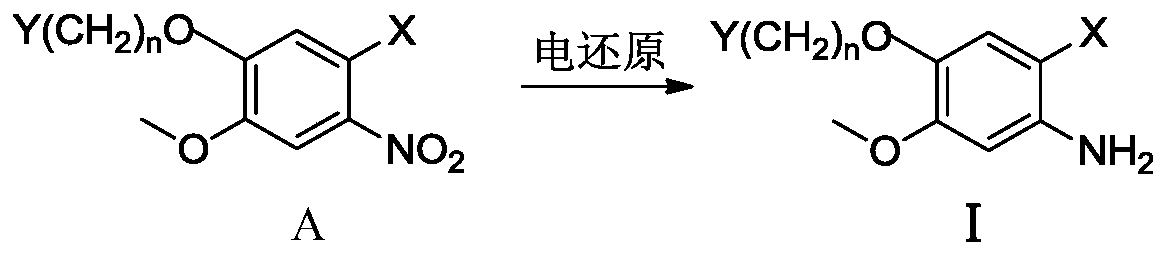
[0077] Electroreduction Preparation of Methyl 2-Amino-4-Methoxy-5-(3-Chloropropoxy)benzoate
[0078]
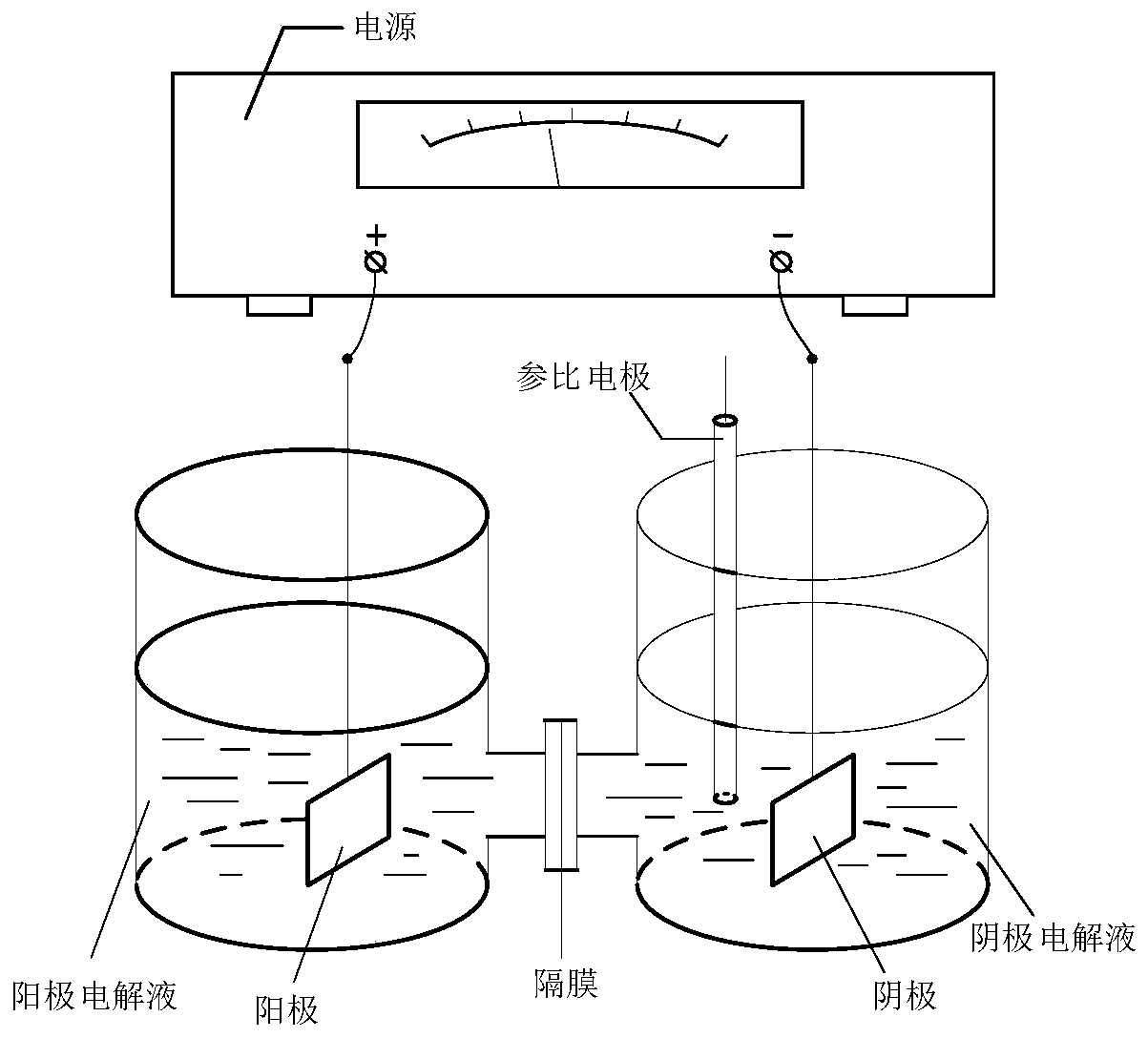
[0079] Install separate electrolyzers ( figure 1 ). A magnetic stirrer was added to the cathode (Cu) electrolyzer, and 0.45g of methyl 4-methoxy-5-(3-chloropropoxy)-2-nitrobenzoate was completely dissolved in 60mL of methanol, and then 60mL of 0.5 mol / L hydrochloric acid solution, or 6.0g ammonium chloride and 60mL water solution, add 120mL 0.25mol / L sulfuric acid solution to the anode (DSA) electrolyzer. The effective electrode area of the cathode (Cu) and anode (DSA) is 4 cm each 2 , control the current at both ends of the anode and cathode to 0.3A, constant current electrolysis, the current density of the constant current is 75mA / cm 2 , the voltage between the cathode and the reference is between 1.1 and 1.4V; stir the electroreduction reaction in a water bath at 40°C for 4 hours, take out the cathode solution, adjust it to weak alkalinity with 10% NaOH solution, e...
Embodiment 2
[0080] Embodiment 2 (control experiment)
[0081] Preparation of methyl 2-amino-4-methoxy-5-(3-chloropropoxy)benzoate
[0082]
[0083] According to the method of literature [Bioorg Med Chem,2010,18(11):3812-3822], adopt iron powder and acetic acid to reduce 4-methoxy-5-(3-chloropropoxy)-2-nitrobenzoic acid methyl ester to obtain methyl 2-amino-4-methoxy-5-(3-chloropropoxy)benzoate, melting point: 96-98°C, yield 77%.
Embodiment 3
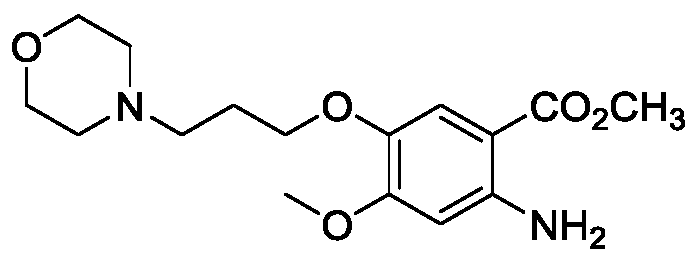
[0085] Preparation of 2-amino-4-methoxy-5-(3-morpholinopropoxy)methyl benzoate by electroreduction
[0086]
[0087] figure 1 Diaphragm type electrolyzer as shown, add magnetic stirrer bar, 0.50g 4-methoxy-5-(3-morpholinopropoxy)-2-methyl nitrobenzoate and 120mL 0.5mol / L hydrochloric acid solution, stir to dissolve it completely, add 120mL 0.25mol / L sulfuric acid solution into the anode (DSA) electrolyzer. The cathode uses a saturated calomel electrode (SCE) as a reference electrode, controls the constant voltage between the cathode and the reference to 2.0V, and the effective electrode area of the cathode (Cu) and anode (DSA) is 4cm each 2 , current density at 60mA / cm 2 Stir the electroreduction reaction in a water bath at 40°C for 3.0 hours, take out the cathode solution, adjust it to weak alkalinity with 10% NaOH solution, extract it twice with dichloromethane, dry it with anhydrous sodium sulfate, filter it with suction, remove the solvent, and dry it to obtain 0.45...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com