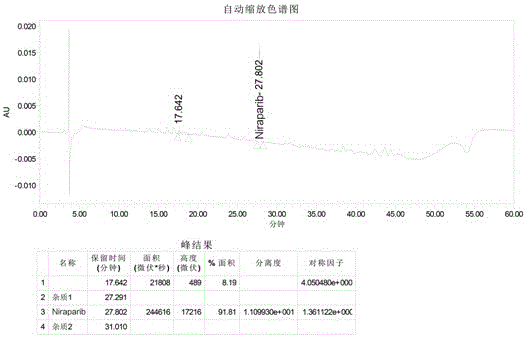
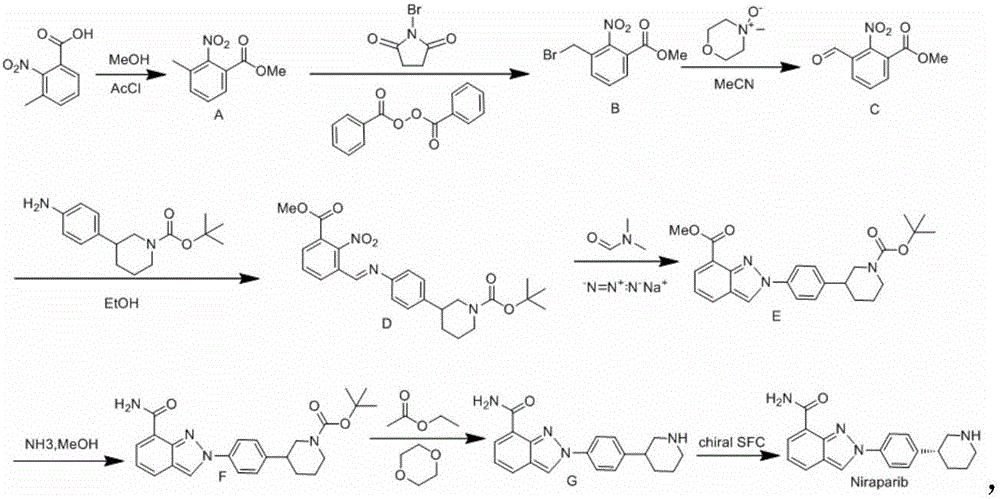
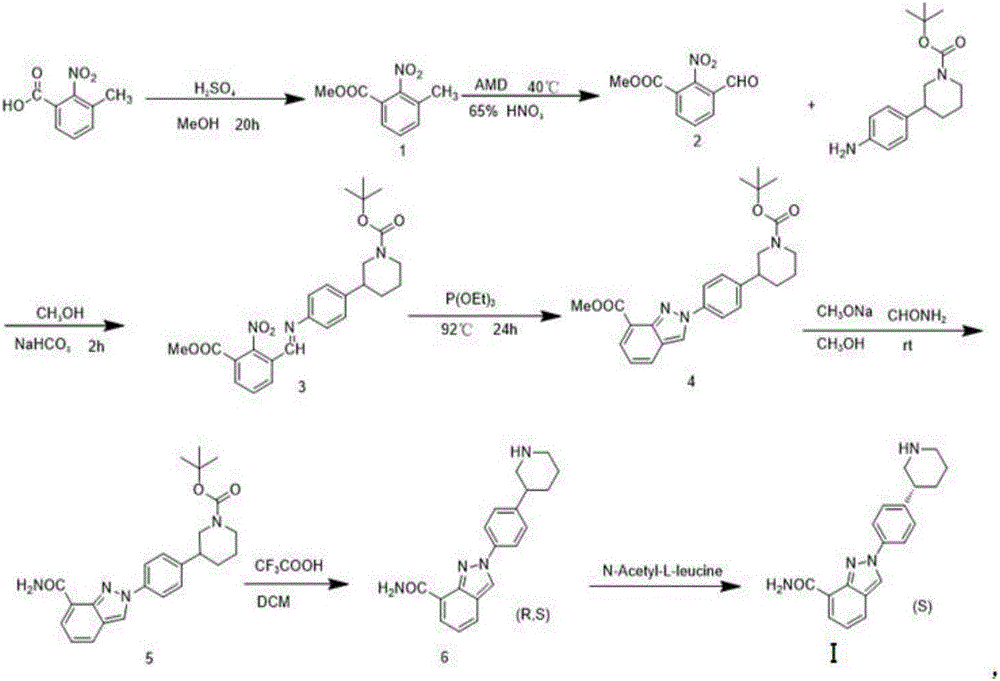
Niraparib synthesis method
A synthetic method and synthetic route technology, applied in the field of synthesis of Niraparib, can solve problems such as difficult to realize large-scale industrial production, limit industrial safety production, long synthetic route, etc., achieve large-scale production, easy large-scale production, The effect of easy control of operating conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Niraparib intermediate 1 Synthesis
[0038]The synthesis of Niraparib intermediate 1 is carried out in two steps, the first step (esterification): 3-methyl-2-nitrobenzoic acid and methyl alcohol are pressed by n (3-methyl-2-nitrobenzoic acid): The ratio of n (methanol) = 1:5, that is, take 182g of 3-methyl-2-nitrobenzoic acid, place 160.2g of methanol in a conical flask with a reflux device, heat slowly, and wait for 3-methyl-2- After the nitrobenzoic acid was completely dissolved in methanol, 6 mL of concentrated sulfuric acid was added, and the temperature was raised to 58°C for the reaction. The product was methyl 3-methyl-2-nitrocarboxylate (Niraparib intermediate 1) and water. The generated Niraparib intermediate 1 is subjected to the second step (refinement and extraction): after the esterification reaction is completed, after the temperature of the reaction solution drops to room temperature, adjust the pH of the solution to neutral with sodium bicarbonate, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com