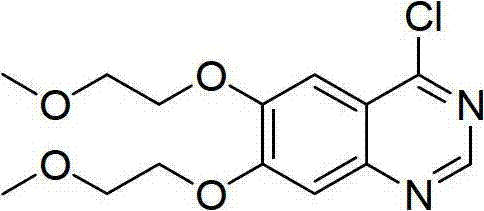
Preparation method for 4-chloro-6,7-bis(2-methoxyethoxy)quinazoline
A technology of methoxyethoxy and quinazoline, which is applied in the field of drug preparation, can solve the problems of high price of ethyl 3,4-dihydroxybenzoate, difficulty in obtaining raw materials, high production cost, etc., and achieve good industrial application Prospects, raw materials and reagents are cheap and easy to obtain, and the effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
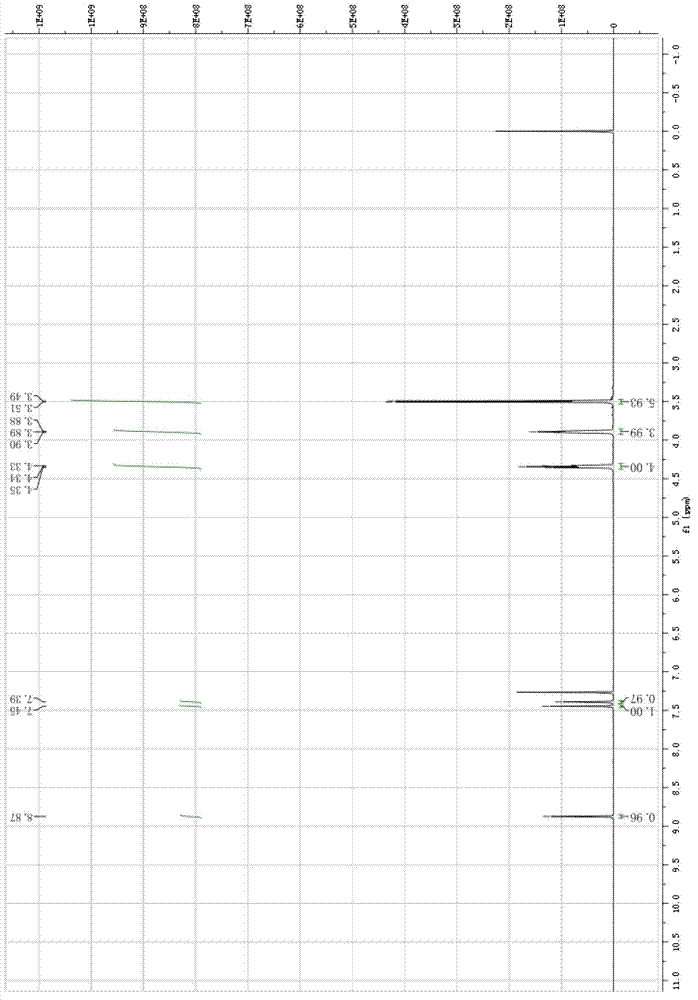
[0040] The raw material 3,4-bis-(2-methoxyethoxy)-benzaldehyde used in the present invention can be prepared by 3,4-dihydroxybenzaldehyde according to literature [Heterocyces, 2007,71 (1), 39-48] Easy to make. The steps of the preparation method of 4-chloro-6,7-bis-(2-methoxyethoxy)-quinazoline are as follows:
[0041] a. Dissolve 3,4-bis-(2-methoxyethoxy)-benzaldehyde in an organic solvent, add concentrated nitric acid with a concentration of 40-90% by mass at 0°C to 20°C, and then React at 20-80°C for 3-10 hours. After the reaction is completed, add water, extract with toluene, wash, dry, and concentrate to obtain 4,5-bis-(2-methoxyethoxy)-2-nitrobenzaldehyde. The molar ratio of concentrated nitric acid to 3,4-di-(2-methoxyethoxy)-benzaldehyde is 1~10:1;
[0042] b. 4,5-bis-(2-methoxyethoxy)-2-nitrobenzaldehyde dissolved in an organic solvent, at 20-80°C and with a mass percent concentration of 10-50% permanganese Potassium acid aqueous solution was reacted for 2 to 10 ho...
Embodiment 1
[0055] (1) Preparation of 4,5-bis-(2-methoxyethoxy)-2-nitrobenzaldehyde
[0056] Add 180g (0.708mol) 3,4-bis-(2-methoxyethoxy)-benzaldehyde, 200ml glacial acetic acid to a 2000ml reaction flask, add 206ml (2.975mol) 65% concentrated nitric acid dropwise at 15°C, After dropping, gradually raise the temperature to 50°C and react for 6 hours. After the reaction is completed, cool to room temperature, add 500ml of water to the reaction bottle, extract twice with 500ml of toluene, and wash the separated toluene layer twice with 1mol / L sodium carbonate aqueous solution. Dry over sodium sulfate, evaporate the solvent, and recrystallize the residue with 1:1 methanol-water to obtain bright yellow crystal 4,5-bis-(2-methoxyethoxy)-2-nitrobenzaldehyde 180g, yield 85%.
[0057] (2) Preparation of 4,5-bis-(2-methoxyethoxy)-2-nitrobenzoic acid
[0058] Add 180g (0.6mol) of 4,5-bis-(2-methoxyethoxy)-2-nitrobenzaldehyde and 200ml of acetone into a 2000ml reaction bottle, and add 1000g of hi...
Embodiment 2
[0068] (1) Preparation of 4,5-bis-(2-methoxyethoxy)-2-nitrobenzaldehyde
[0069] Add 180g (0.708mol) 3,4-bis-(2-methoxyethoxy)-benzaldehyde and 200ml propionic acid to a 2000ml reaction flask, add 49ml (0.708mol) 65% concentrated nitric acid dropwise at 15°C, After dropping, gradually raise the temperature to 50°C and react for 6 hours. After the reaction is completed, cool to room temperature, add 500ml of water to the reaction bottle, and extract twice with 500ml of toluene. The separated toluene layer is washed twice with 1mol / L sodium carbonate solution. Dry over sodium sulfate, evaporate the solvent, and recrystallize the residue with 1:1 methanol-water to obtain 105.8 g of bright yellow crystal 4,5-bis-(2-methoxyethoxy)-2-nitrobenzaldehyde. The rate is 50%.
[0070] (2) Preparation of 4,5-bis-(2-methoxyethoxy)-2-nitrobenzoic acid
[0071] Add 105g (0.35mol) of 4,5-bis-(2-methoxyethoxy)-2-nitrobenzaldehyde and 200ml dioxane to a 2000ml reaction bottle, dissolve and add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com