SFM (serum-free medium) for culturing MSCs (mesenchymal stem cells)
A serum-free medium and quality stem cell technology, applied in the field of serum-free medium, can solve the problems of increasing the chance of cell culture workload contamination, high difficulty in quality control, and difficulty in standardizing cells, so as to increase the chance of contamination and the complexity of operation. The effect of ensuring the stability of batches and avoiding heterologous contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
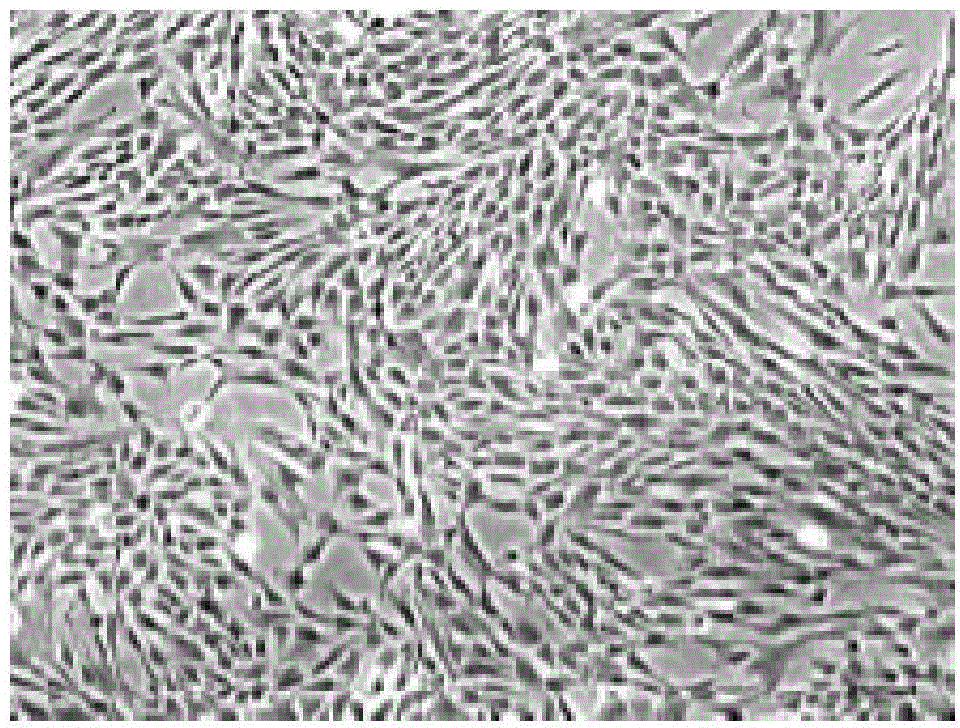
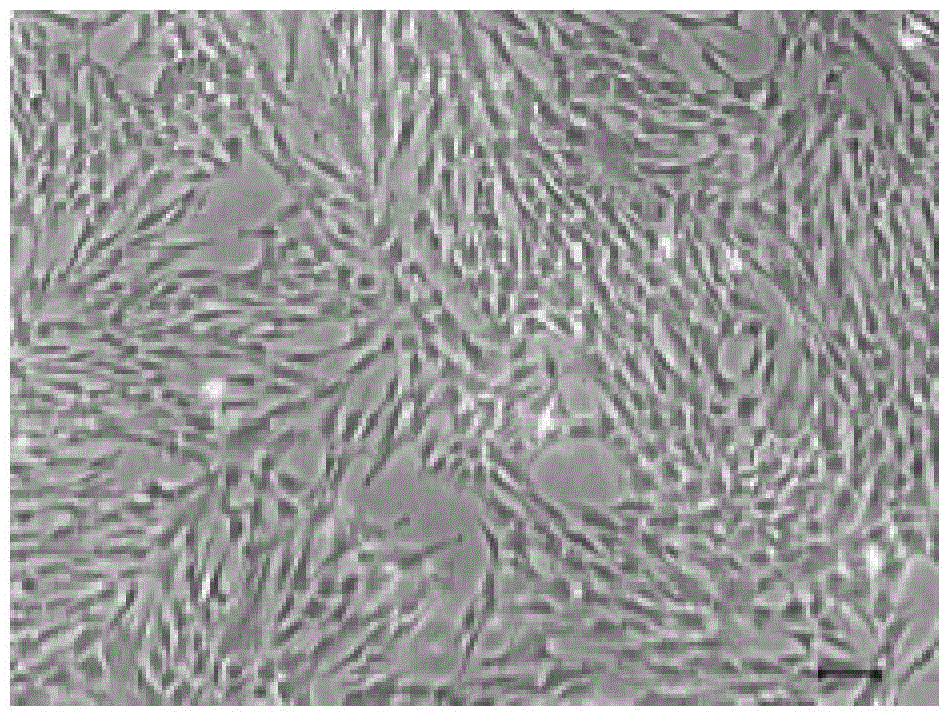
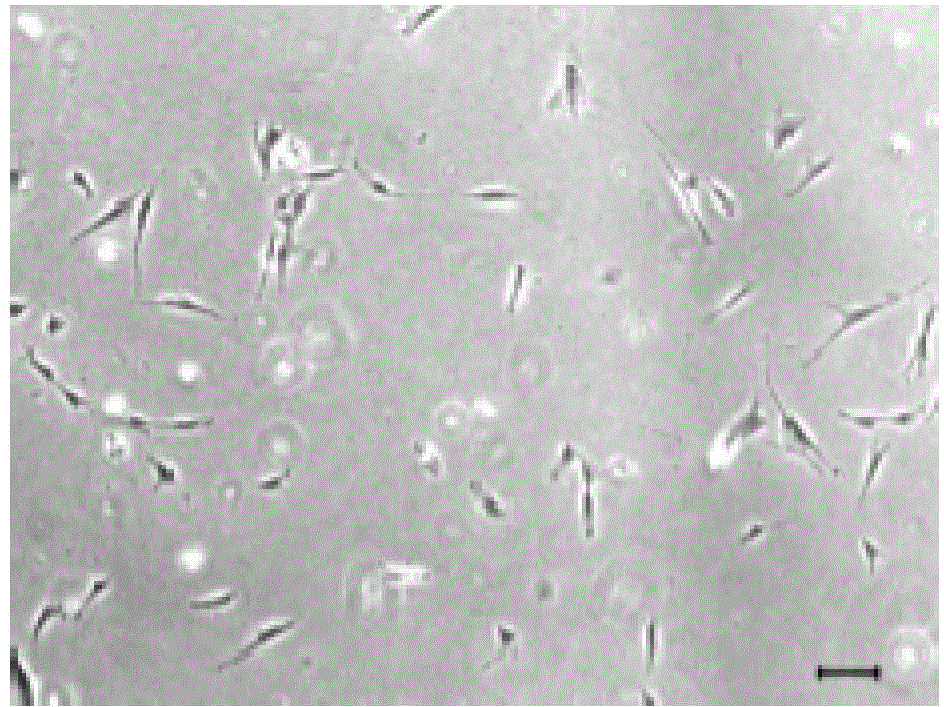
Image
Examples
Embodiment 1
[0041] This example provides a BPS-SFM medium, the specific components of which are shown in Table 2:
[0042] Table 2
[0043] Component
Content
α-MEM
10.2g / L
2.4g / L
[0044] L-glutamine
5mM
Poloxamer 188
100mg / L
Recombinant Human Albumin
8g / L
Recombinant human transferrin
20mg / L
Recombinant human insulin
10mg / L
5mM
β-mercaptoethanol
50nM
0.5mM
50nM
0.26mg / L
0.25mg / L
0.28mg / L
0.28mg / L
0.28mg / L
0.28mg / L
Vitamin PP
50mg / L
Vitamin C
20mg / L
Cu
5nM
Se
30nM
Zn
1mM
Ga
0.3mM
Cr
5μM
Mg
0.3mM
Mn
5nM
1mg / L
1mg / L
50ng / mL
Compound of formula I
10μM
Compound represented by formula II
20μM
Progesterone
15ng / mL
10mg / L
10IU / mL
EGF
10ng / mL
b-FGF
10ng / mL
HGF
10ng / mL
VEGF
10ng / mL
...
Embodiment 3
[0059] Example 3 Detection of cell proliferation ability
[0060] In this example, the cell proliferation ability of the BPS-SFM medium provided in Example 1 and the control group were compared and tested, specifically according to the following steps:
[0061] Treat the P4 generation umbilical cord mesenchymal stem cells in two different serum-free media of the BPS-SFM medium and the control group, respectively, with 4×10 4 / mL and 6×10 4 Inoculate a 96-well plate at a density of 100 μL per well, and inoculate 5 parallel wells for each group of cells; add CCK-8 reagent at a ratio of 1:10 after the cells adhere to the wall for 24 hours at 37℃, 5% CO 2 Incubate for 2h in the incubator, and measure the OD value.
[0062] See the cell proliferation results Figure 2a with Figure 2b Shown, where, Figure 2a Is 4×10 4 The cell proliferation results of the two when inoculated at a density of / mL, Figure 2b Is 6×10 4 The cell proliferation results of both when inoculated at a density of / m...
Embodiment 4
[0063] Example 4 Detection of cell phenotype by flow cytometry
[0064] In this example, the cell phenotype of the P4 generation cells in the BPS-SFM medium provided in Example 1 and the control group MSCM-SF was performed according to the following steps:
[0065] TrypLE was used to digest and collect the P4 generation cells in the experimental group BPS-SFM medium and the control group MSCM-SF. They were incubated with fluorescently-labeled CD29, CD90, CD105, CD34 and CD45 and other surface antibodies, and were incubated with FITC and PE-labeled small cells. Mouse IgG isotype antibody was used as a control; incubate at 4°C for 45min and centrifuge to collect the cells; after washing 3 times with PBS buffer, resuspend the cells in 400μL PBS buffer and perform flow cytometry analysis on the machine. The specific results are as follows Table 3 shows.
[0066] table 3
[0067]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Osmotic pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com