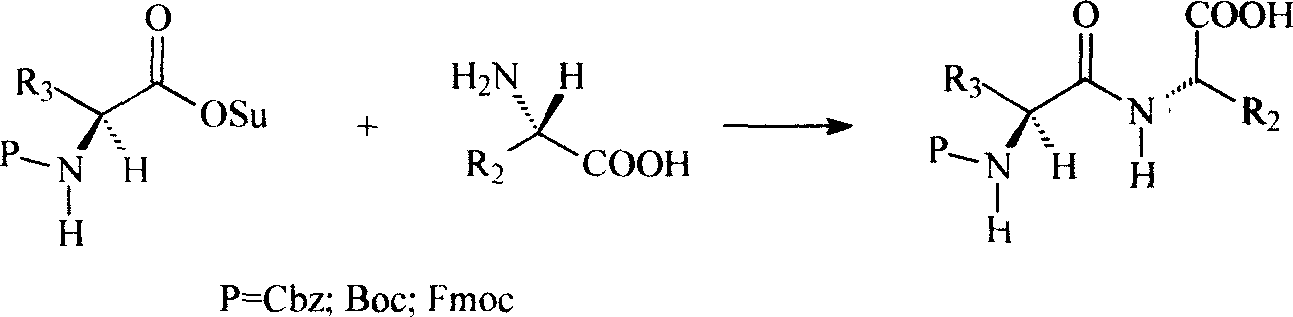
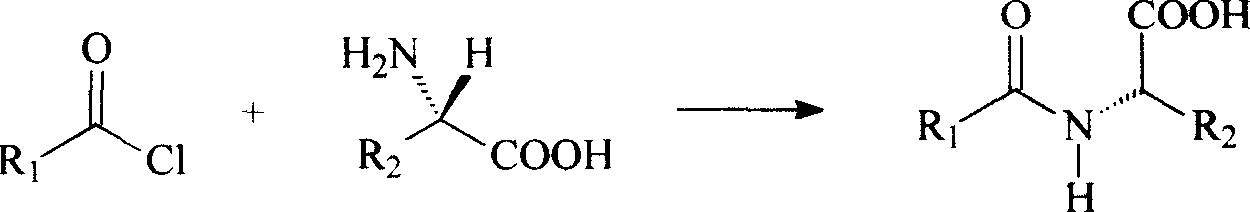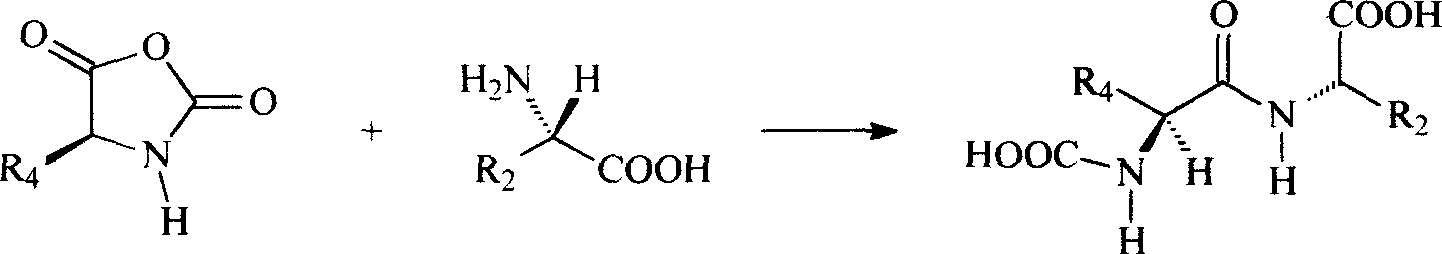Preparation method of N(2)-L-alanyl-L-glutamine
A technology of glutamine and alanyl, which is applied in the field of preparation of N-L-alanyl-L-glutamine, can solve the problems of high price, uneconomical dipeptide and high raw material price, and achieves convenient operation and less pollution , the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation method of N(2)-L-alanyl-L-glutamine of the present invention is as follows, and its steps comprise:
[0034] 1. Preparation of potassium precursor acid
[0035] In the reactor for preparing potassium precursor acid, add 32.5g KOH, 45.0g alanine and 100ml ethanol respectively; stir at room temperature for 10 minutes, dissolve, and obtain a clear solution; then add 90.0ml ethyl acetoacetate dropwise, and reflux for 60 Minutes, the temperature in the reactor is at 68°C;
[0036] Change back distillation to distillation to recover ethanol. When the temperature in the reactor is 88°C, add 150ml of toluene to continue distillation. When the temperature in the reactor is 78°C, add 100ml of toluene, stir and distill 50ml of toluene. Distill 250ml of toluene, stir back to distill with water, distill 130ml of toluene and water in total, separate 23ml of water, stir and distill until the temperature in the reactor reaches 110°C, and crystals are precipitated, and ...
Embodiment 2
[0050] The preparation method of N(2)-L-alanyl-L-glutamine of the present invention is as follows, and its steps comprise:
[0051] 1. Preparation of potassium precursor acid
[0052] In the reactor for preparing potassium precursor acid, add 32.5g KOH, 45.0g alanine and 100ml ethanol respectively; stir at room temperature for 10 minutes, dissolve, and obtain a clear solution; then add dropwise 73.0ml acetylacetone, and reflux for 60 minutes, The temperature in the reactor is 84°C;
[0053] Change back distillation to distillation to recover ethanol. When the temperature in the reactor is 88°C, add 150ml of toluene to continue distillation. When the temperature in the reactor is 78°C, add 100ml of toluene, stir and distill 50ml of toluene. Distill 250ml of toluene, stir back to distill with water, distill 130ml of toluene and water in total, separate 23ml of water, stir and distill until the temperature in the reactor reaches 110°C, and crystals are precipitated, and cool at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com