N(2)-L-alanyl-L-glutamine/compound amino acid injection (18AA-V) pharmaceutical composite preparation
A 18AA-V, compound amino acid technology, applied in the directions of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of not exceeding 3.5%, failing to meet clinical requirements, etc., achieving less adverse reactions, good application prospects, and scope of application wide effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
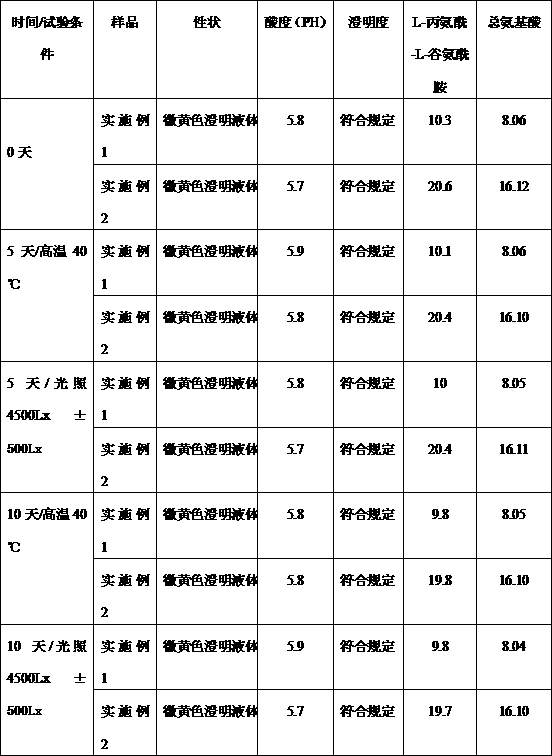
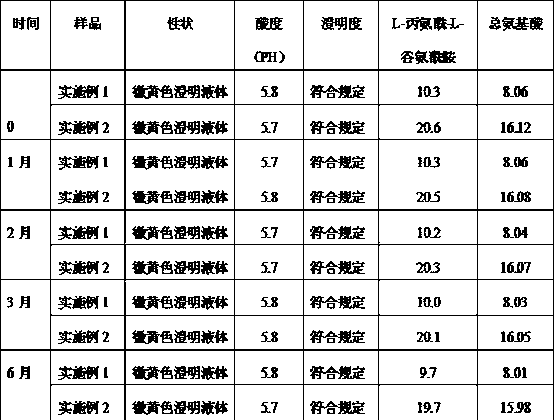
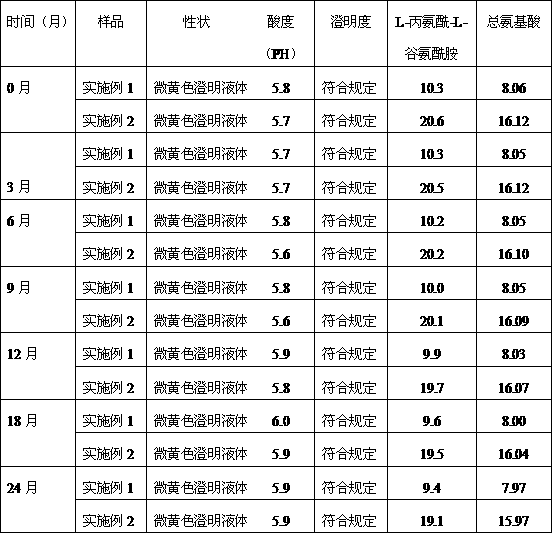
[0012] N(2)-L-alanyl-L-glutamine / compound amino acid injection (18AA-V) drug combination preparation is made of the following components by weight:
[0013] N(2)-L-alanyl-L-glutamine for injection 10g;
[0014] Sterile water for injection 50ml;
[0015] Compound amino acid injection (18AA-V) 250ml: 8.06g (total amino acid) and 12.5g xylitol.
Embodiment 2
[0017] N(2)-L-alanyl-L-glutamine / compound amino acid injection (18AA-V) drug combination preparation is made of the following components by weight:
[0018] N(2)-L-alanyl-L-glutamine for injection 20g;
[0019] Sterile water for injection 100ml;
[0020] Compound amino acid injection (18AA-V) 500ml: 16.12g (total amino acid) and 25g xylitol.
[0021] Quality Research Survey
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com