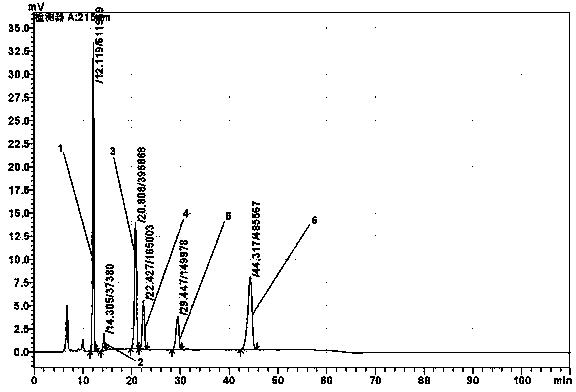
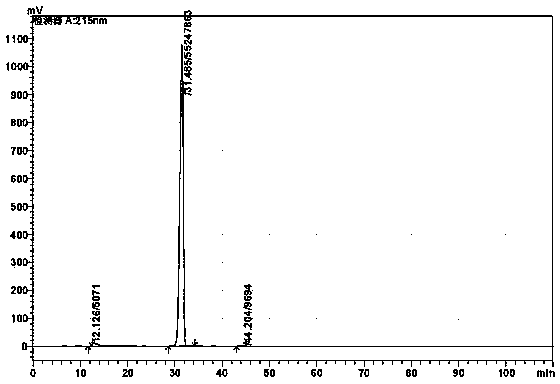
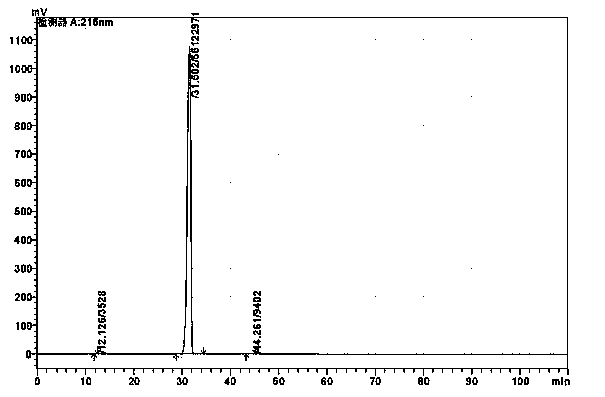
Refining method of alanyl-glutamine crude drug
A technology of alanyl glutamine and refining method, which is applied in the fields of peptide preparation method, chemical instrument and method, organic chemistry, etc. It can solve the problem of fine-quality products without adjusting the pH value, not being able to be directly prepared by injection, and the content of fine-quality products To achieve the effect of reducing the possibility of new impurities, reducing the content of impurities and bacterial endotoxin, and moderate crystallization speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 50kg of alanylglutamine into 150kg of water, stir until completely dissolved, add 5% potassium hydroxide solution to adjust the pH to 5.0, then add 0.25kg of activated carbon, keep the stirring speed at 200 rpm, stir and absorb at room temperature for 30min, filter and decolorize to obtain Colorless clear liquid. Add the clarified feed liquid into the crystallization tank and raise the temperature to 40°C. Maintain the stirring speed at 200 rpm, add methanol 600kg at 45°C, and white crystals precipitate out. After the precipitation is completed, they are centrifuged and washed, the filter cake is vacuum-dried, and 47.5kg of high-purity alanyl glutamine is obtained by weighing, with a yield of 95%. 99.97% (HPLC detection).
[0034] HPLC impurity reference substance method detection, cyclo-(L-alanyl-L-glutamine), cyclo-(L-alanyl-L-glutamyl), L-pyroglutamyl-L-alanine Acid, L-pyroglutamic acid, D-alanyl-L-glutamine (or L-alanyl-D-glutamine), L-alanyl-L-glutamic acid a...
Embodiment 2
[0044] Add 100kg of alanylglutamine into 500kg of water, stir until completely dissolved, add 10% potassium hydroxide solution to adjust the pH to 5.5, then add 0.3kg of activated carbon, maintain the stirring speed at 400 rpm, stir and absorb at 50°C for 30 minutes, filter and decolorize A colorless clear liquid was obtained. Add the clarified feed liquid into the crystallization tank and raise the temperature to 45°C. Maintain the stirring speed at 500 rpm, add 1280kg of methanol at 62°C, and white crystals precipitate out. After the precipitation is completed, they are centrifuged and washed, the filter cake is vacuum-dried, and the high-purity alanyl glutamine is weighed to obtain 95kg. The yield is 95%, and the purity is 99.98. % (HPLC detection).
[0045] HPLC impurity reference substance method detection, cyclo-(L-alanyl-L-glutamine), cyclo-(L-alanyl-L-glutamyl), L-pyroglutamyl-L-alanine Acid, L-pyroglutamic acid, D-alanyl-L-glutamine (or L-alanyl-D-glutamine), L-alan...
Embodiment 3
[0053] Add 100kg of alanylglutamine into 250kg of water, stir until completely dissolved, add methanol solution with a potassium methoxide content of about 28%-32%, adjust the pH to 6.0, then add 0.5kg of activated carbon, maintain the stirring speed at 500rpm, 60℃ Stir and adsorb for 30 minutes, filter and decolorize to obtain a colorless and clear feed liquid. Add the clarified feed liquid into the crystallization tank and raise the temperature to 50°C. Maintain the stirring speed at 400 rpm, add 1000 kg of methanol at 40 °C, white crystals precipitate out, centrifuge and wash the filter cake after the precipitation, vacuum dry the filter cake, weigh 95 kg of high-purity alanyl glutamine, yield 95%, purity 99.97 % (HPLC detection).
[0054] HPLC impurity reference substance method detection, cyclo-(L-alanyl-L-glutamine), cyclo-(L-alanyl-L-glutamyl), L-pyroglutamyl-L-alanine Acid, L-pyroglutamic acid, D-alanyl-L-glutamine (or L-alanyl-D-glutamine), L-alanyl-L-glutamic acid ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com