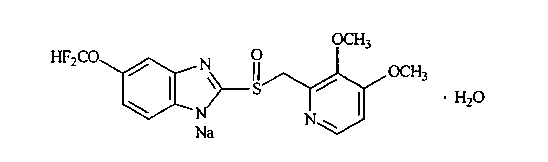
Levo-pantoprazole salt aquo-complex enteric-coated tablet and preparation method thereof
A technology for pantoprazole salts and hydrates, applied in the field of enteric-coated tablets and their preparation, can solve the problems of being easily affected by various environmental factors, being difficult to develop, and being unstable, so as to avoid adverse reactions and achieve stable quality. Reliable and uniform results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
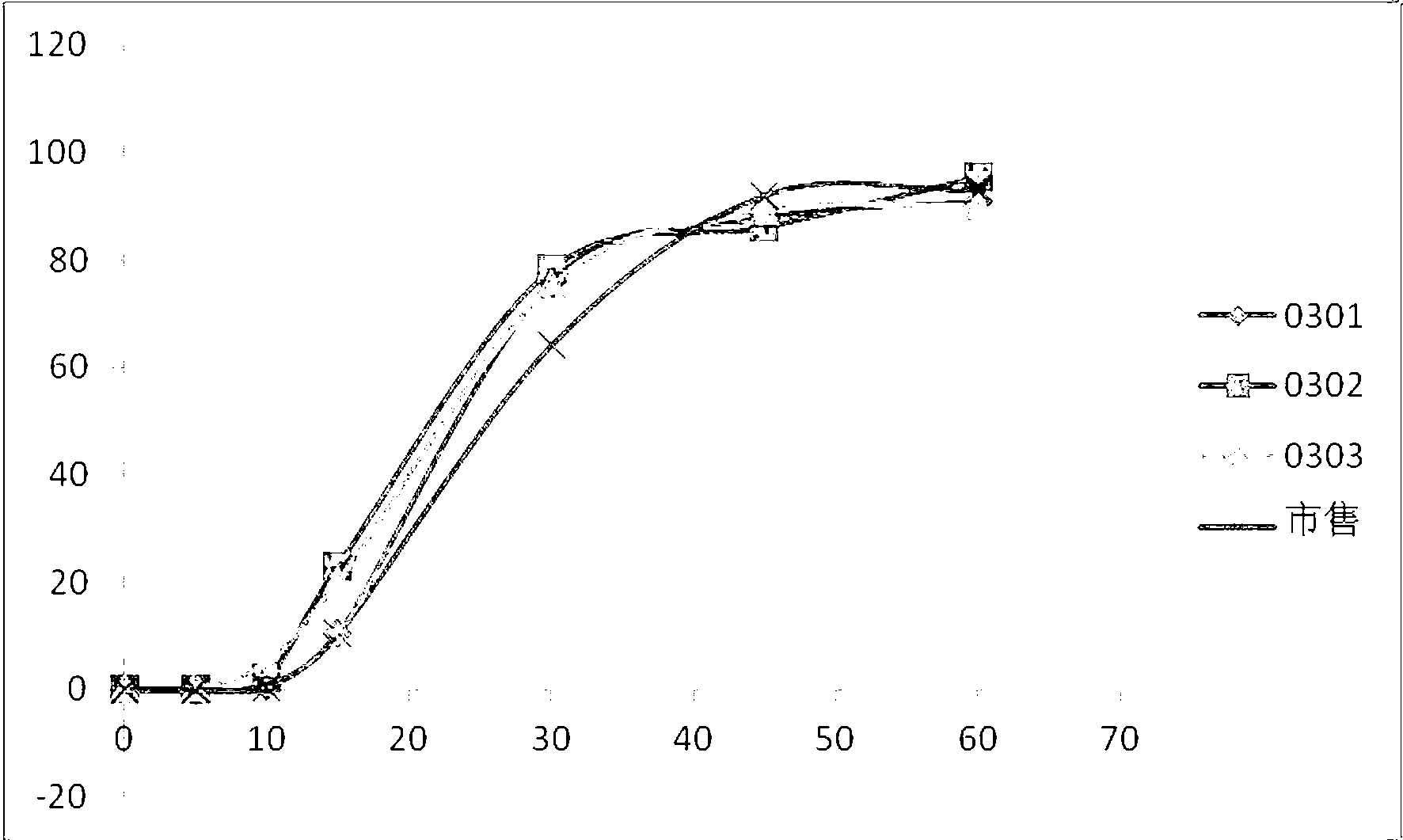
Image
Examples
Embodiment 1
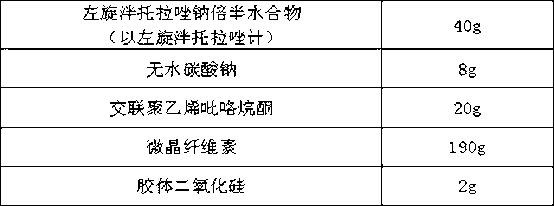
[0040] A levo-pantoprazole salt hydrate enteric-coated tablet is characterized in that: 40 g of le-pantoprazole sodium sesquihydrate (calculated as le-pantoprazole), 10 g of anhydrous sodium carbonate, cross-linked polyethylene 20g of pyrrolidone, 188g of microcrystalline cellulose, 2g of colloidal silicon dioxide, 25g of Opadry (03K19229) (separation layer) and 28.6g of Acryl (93F19255) (enteric-coated layer) were prepared into 2000 tablets.
[0041] A kind of preparation method of L-pantoprazole salt hydrate enteric-coated tablet is characterized in that comprising the steps:
[0042] 1) Preparation of tablet core
[0043] Pass the raw and auxiliary materials through a 80-mesh sieve, weigh 40 g of levo-pantoprazole sodium sesquihydrate, 10 g of anhydrous sodium carbonate, and 10 g of cross-linked polyvinylpyrrolidone, mix well, and then add microcrystalline fiber Plain 188g, mixing, dry granulation, add cross-linked polyvinylpyrrolidone 10g and colloidal silicon dioxide 2g,...
Embodiment 2
[0051] A levo-pantoprazole salt hydrate enteric-coated tablet is characterized in that it is composed of 40 g of le-pantoprazole sodium sesquihydrate (calculated as le-pantoprazole), 12 g of anhydrous sodium carbonate, and cross-linked polyvinylpyrrolidone 10g, microcrystalline cellulose 135g, colloidal silicon dioxide 3g, Opadry (03K19229) 8g and Acryl (93F19255) 24g to make 2000 tablets.
[0052] A preparation method for L-pantoprazole salt hydrate enteric-coated tablets, comprising the steps of:
[0053] 1) Preparation of tablet core
[0054] Pass the raw and auxiliary materials through a 80-mesh sieve, weigh 40g of levopantoprazole sodium sesquihydrate hydrate, 12g of anhydrous sodium carbonate and 5g of cross-linked polyvinylpyrrolidone, mix well, and then add microcrystalline Cellulose 135g, mix evenly, dry granulate, add remaining cross-linked polyvinylpyrrolidone 5g and colloidal silicon dioxide 3g, mix uniformly, tabletting, obtain the tablet core of L-pantoprazole s...
Embodiment 3
[0062] A levo-pantoprazole salt hydrate enteric-coated tablet is characterized in that it is composed of 65 g of le-pantoprazole sodium sesquihydrate (calculated as le-pantoprazole), 17 g of anhydrous sodium carbonate, and cross-linked polyvinylpyrrolidone 20g, microcrystalline cellulose 156g, colloidal silicon dioxide 2g, Opadry (03K19229) 16g and Acryl (93F19255) 26g to make 2000 tablets.
[0063] A preparation method for L-pantoprazole salt hydrate enteric-coated tablets, comprising the steps of:
[0064] 1) Preparation of tablet core
[0065] The raw and auxiliary materials are passed through an 80 mesh sieve, 65g of levopantoprazole sodium sesquihydrate hydrate, 17g of anhydrous sodium carbonate and 10g of cross-linked polyvinylpyrrolidone, which are weighed, are mixed, and then added in micro Crystalline cellulose 156g, mixing, dry granulation, add remaining cross-linked polyvinylpyrrolidone 10g and colloidal silicon dioxide 2g, mix homogeneously, compress tablet, obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com