Dihydroartemisinin piperaquine phosphate tablets and preparation process thereof
A technology of dihydroartemisinin and piperaquine, which is applied in the field of dihydroartemisinin-piperaquine tablets and their preparation, can solve the problems of easy discoloration of piperaquine phosphate and the inability of plain tablets to guarantee the stability of medicines, and achieves one-sided bright and clean , less impurities, the effect of overcoming the problem of discoloration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Prepare according to the following formula:
[0041] Dihydroartemisinin (Chongqing Huali Wulingshan Pharmaceutical Co., Ltd.)
40.0g
[0042] Piperaquine Phosphate (Zhuhai Rundu Mintong Pharmaceutical Co., Ltd.)
320.0g
Corn starch (Haiyan Liuhe Starch Chemical Co., Ltd.)
70.6g
Dextrin (Haiyan Liuhe Starch Chemical Co., Ltd.)
17.7g
Hypromellose (Huzhou Zhanwang Pharmaceutical Co., Ltd.)
2.9g
Sodium carboxymethyl starch (Huzhou Zhanwang Pharmaceutical Co., Ltd.)
212g
Magnesium stearate (Huzhou Zhanwang Pharmaceutical Co., Ltd.)
2.18g
Opadry (Shanghai Colorcon Coating Technology Co., Ltd.)
13.4g
production
1000 pieces
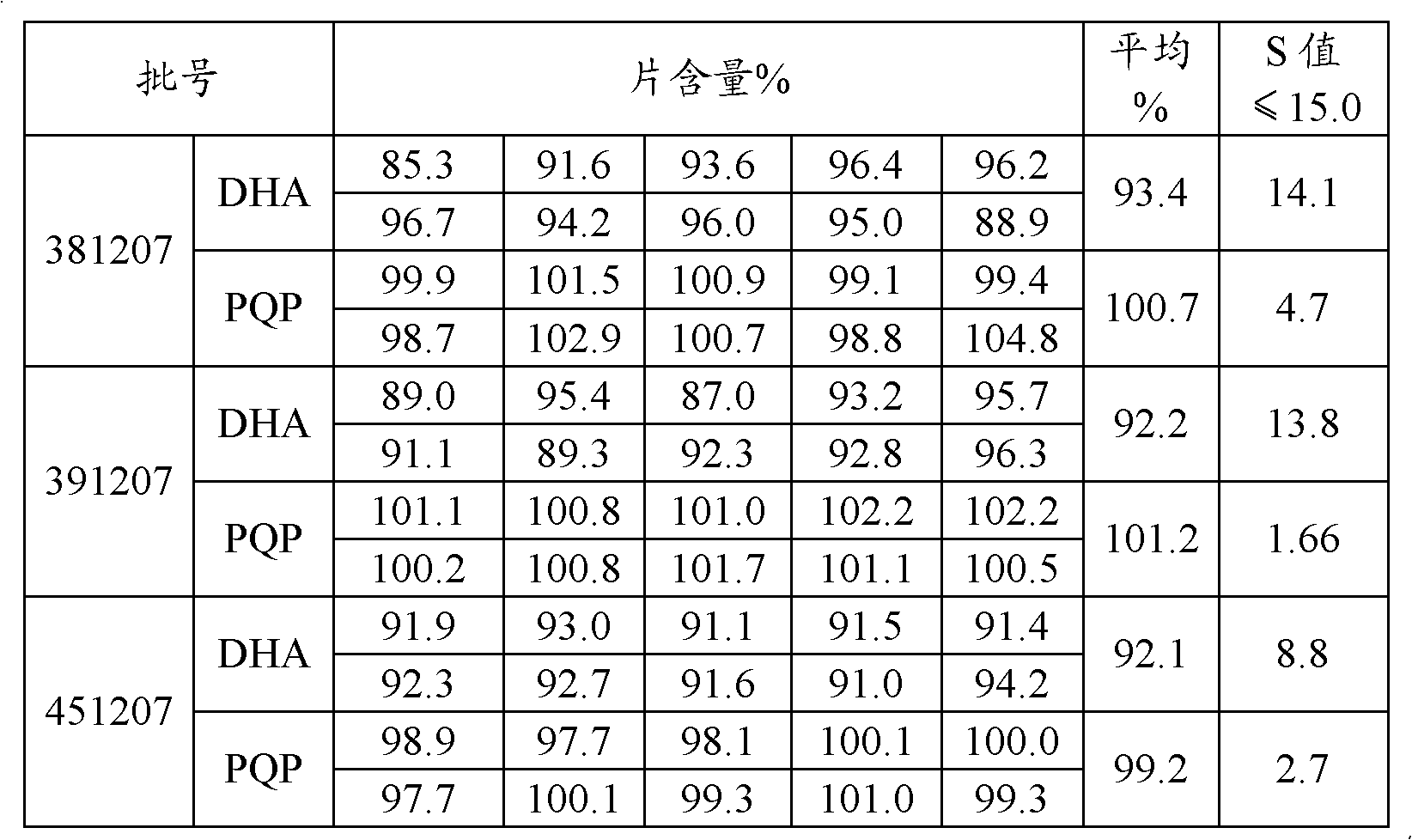
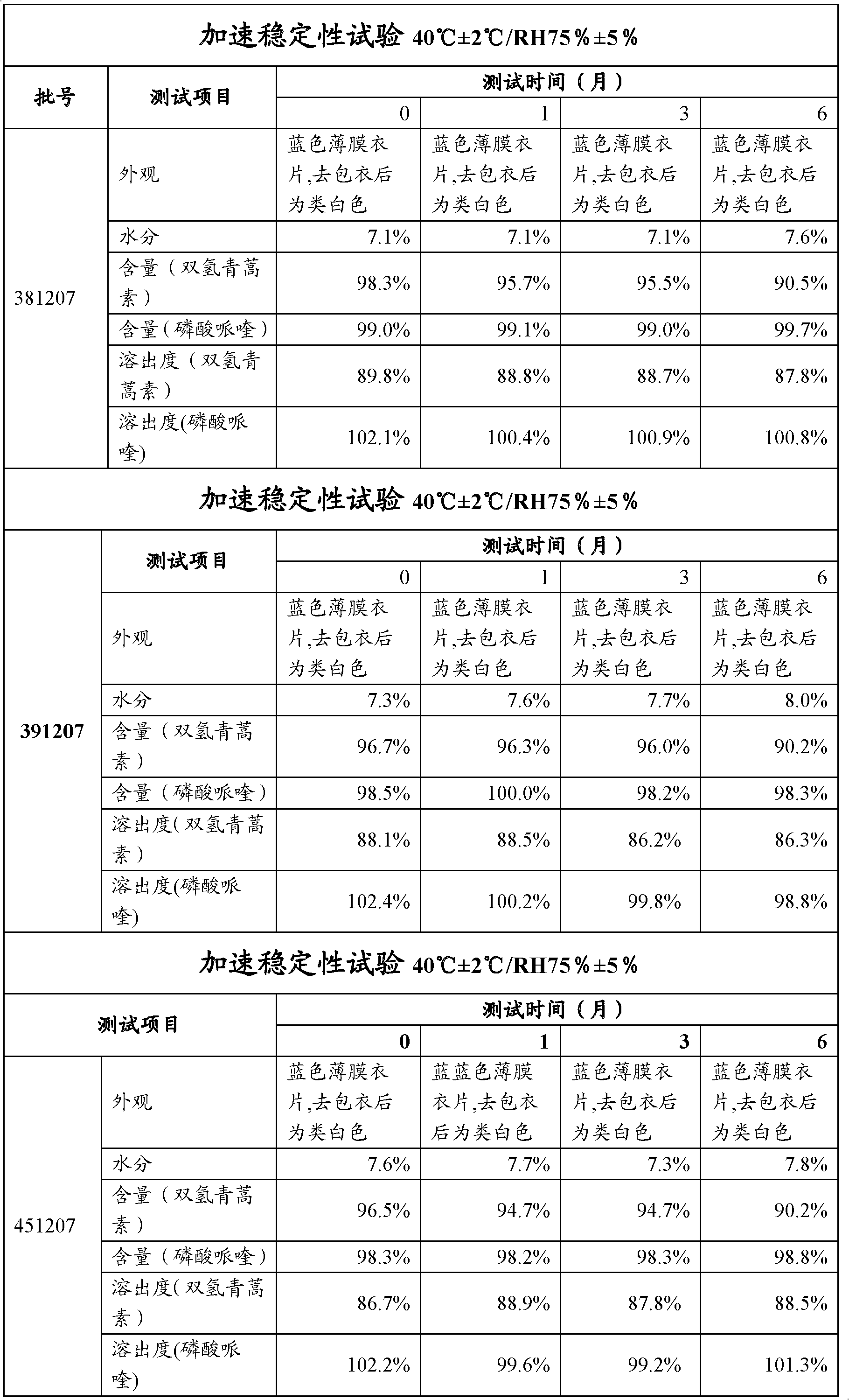
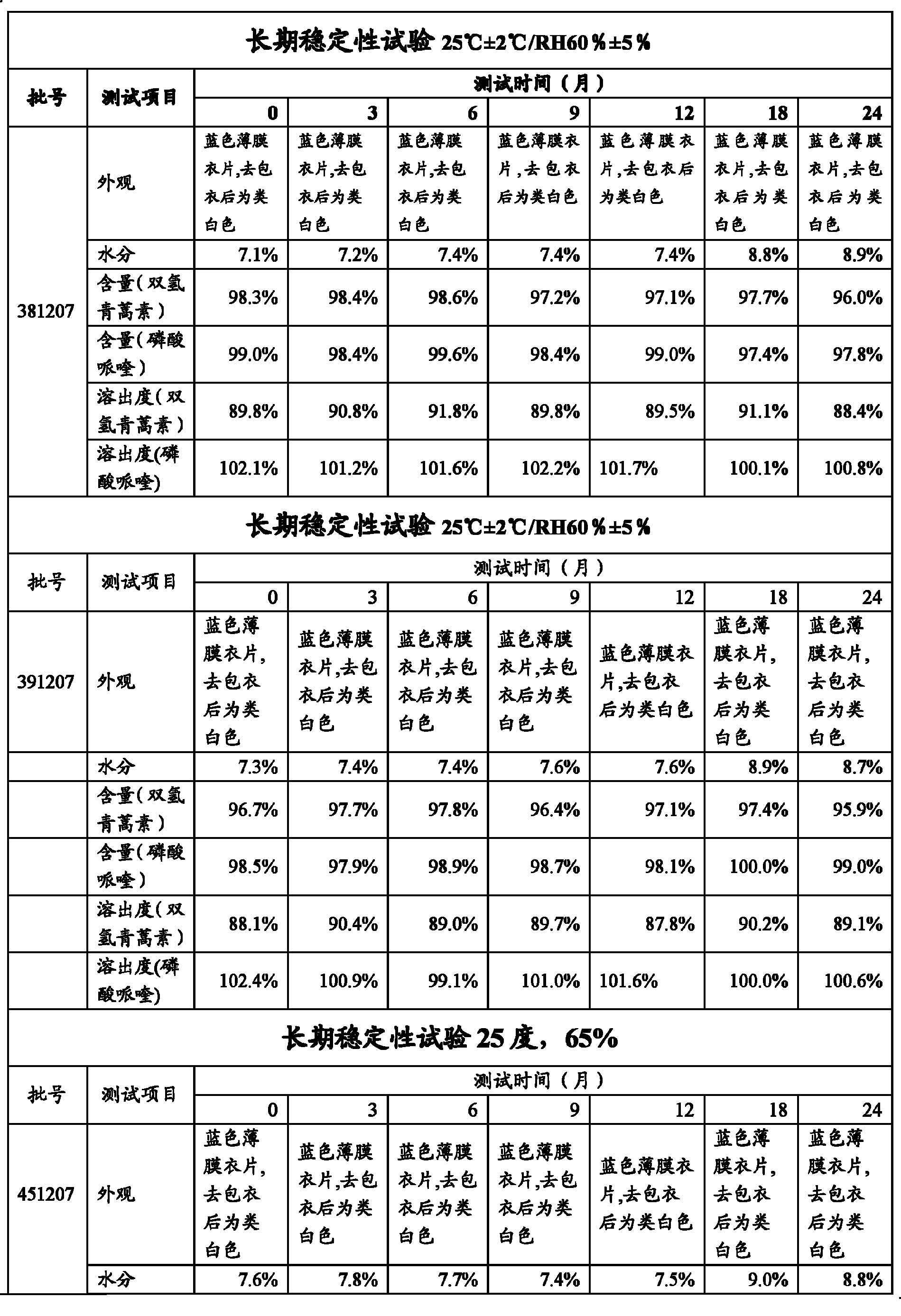
[0043] Grind 40g of double artemisinin and 320g of piperaquine phosphate with a 100-mesh sieve and pass through a 100-mesh steel sieve, requiring all to pass through a 100-mesh sieve; 70.6g of corn starch, 17.7g of dextrin, 2.9g of hypr...
Embodiment 2
[0052] 40g of bisartemisinin and 320g of piperaquine phosphate were crushed with a 100-mesh sieve and passed through a 100-mesh steel sieve, requiring all to pass through a 100-mesh sieve; 80.9g of corn starch, 22.8g of dextrin, 2.9g of hypromellose, hard Magnesium fatty acid 3.5g and sodium carboxymethyl starch 8.6g pass through a 60-mesh steel sieve respectively for later use.
[0053] The hypromellose is dissolved in an ethanol solution with a concentration of 50% by mass, and stirred until dissolved to form a binder with a concentration of 8% by mass.
[0054] Add the above-mentioned piperaquine phosphate, dihydroartemisinin, starch, dextrin and carboxymethyl starch sodium after sieving into the wet granulator, add hypromellose binder to make soft material, pass through a 14-mesh sieve, Get wet granules.
[0055] The wet granules are dried in an explosion-proof boiling dryer, and the material temperature is controlled at 40-50°C to obtain dry granules.
[0056] The dry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com