Sclera external pressurized biologic composite materials and preparation method
A composite material and biological technology, applied in the field of medical biomaterials, can solve the problems of high residual concentration of crosslinking agent, uneven material hardness, failure to meet the requirements, etc., to achieve biocompatibility and mechanical strength, high surface hardness and Effect of impact resistance, good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Use 100g of pharmaceutical grade hyaluronic acid, add water to swell overnight at 4°C, prepare 10% sodium hyaluronate solution in 1000ml for use, prepare crosslinking reaction solution A according to the following formula, and its components and weight percentages:
[0037] BDDE (Butanediol Diglycidyl Ether) 30ml
[0038] Sodium hydroxide solution 0.5mol / L: 50ml
[0039] Distilled water to 500ml
[0040] Add the cross-linking reaction solution A into the hyaluronic acid solution, react at 40°C for 6 hours to obtain the preliminary cross-linking product, wash it repeatedly with distilled water for 4 times, then add the second-step cross-linking solution B, and the second-step cross-linking solution B The content of components and weight percent:
[0041] DVS (Diethylsulfone) 10ml
[0042] Sodium carbonate / sodium bicarbonate solution 0.8mol / L: 50ml pH=10
[0043]Distilled water to 500ml
[0044] Continue to react for 4 hours, wash with distilled water, then add 1000m...
Embodiment 2
[0050] Use 100g of pharmaceutical grade hyaluronic acid, add water to swell overnight at 16°C, prepare a 500ml solution of 20% sodium hyaluronate for use, prepare a cross-linking reaction solution A, and its components and weight percentages are as follows:
[0051] Formaldehyde solution 50ml
[0052] Sodium hydroxide solution 0.5mol / L: 40ml pH=12
[0053] 95% ethanol 20ml
[0054] Distilled water to 500ml
[0055] Add the cross-linking reaction solution A into the hyaluronic acid solution, and react at 4°C for 2 hours to obtain the preliminary cross-linking product, wash it repeatedly with distilled water, then add the cross-linking reaction solution B, the components and weight of the cross-linking reaction solution B Percent content:
[0056] Bissulfosuccinimide suberate (BS3) 5ml
[0057] 0.5N phosphate buffer solution 100ml pH=5.0
[0058] 95% ethanol 10ml
[0059] Distilled water to 500ml
[0060] Continue to react at 4°C for 3 hours, wash with distilled water, th...
Embodiment 3
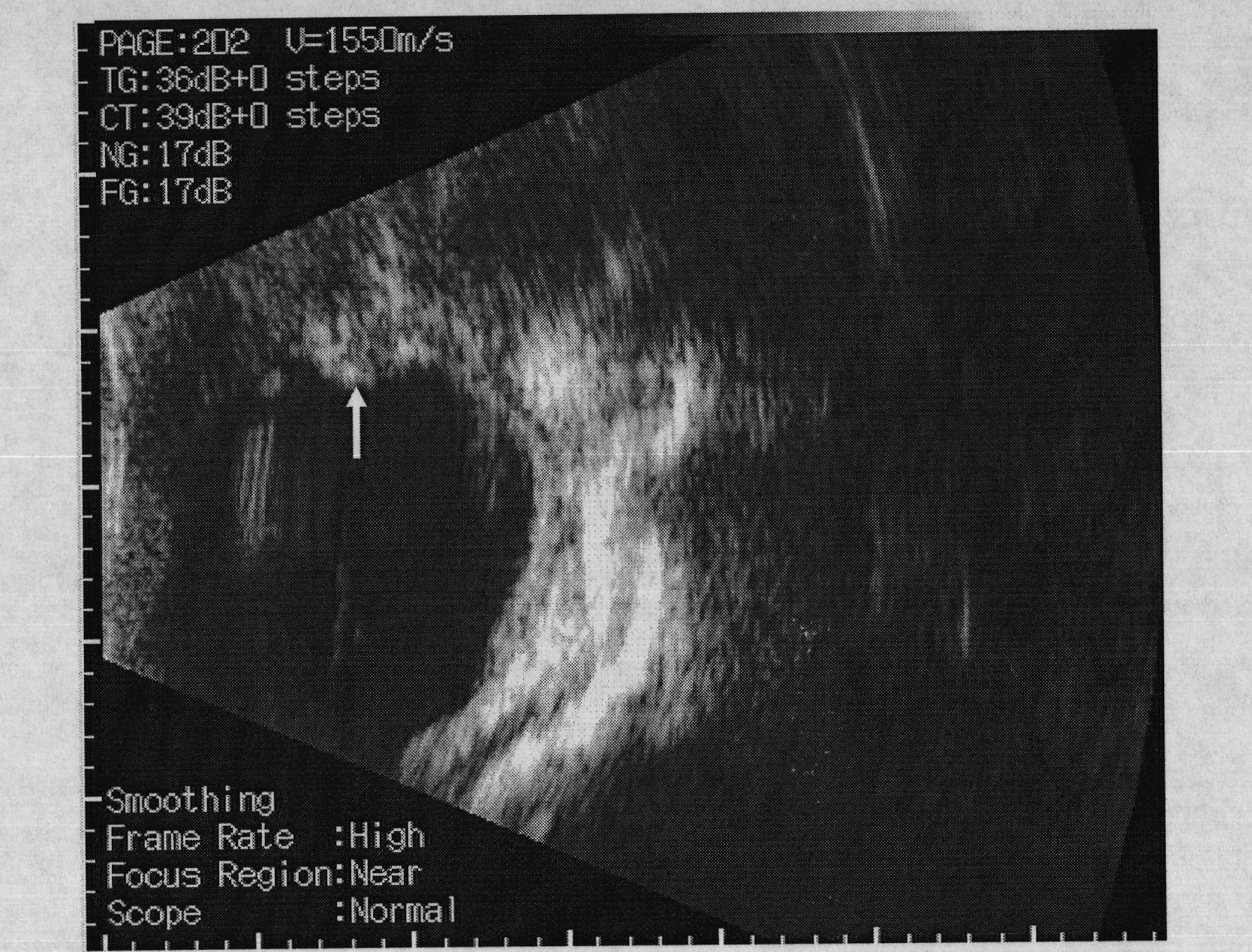
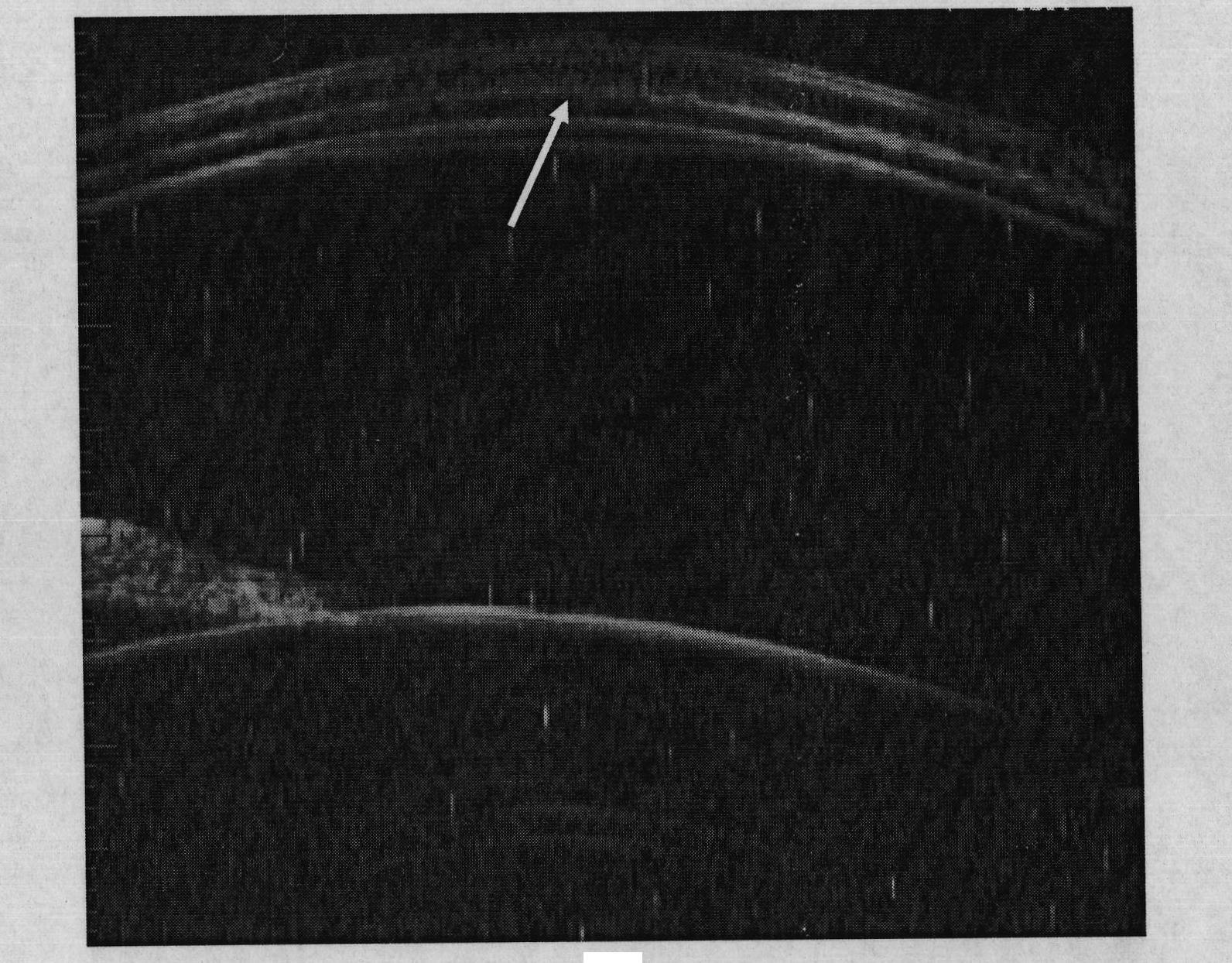
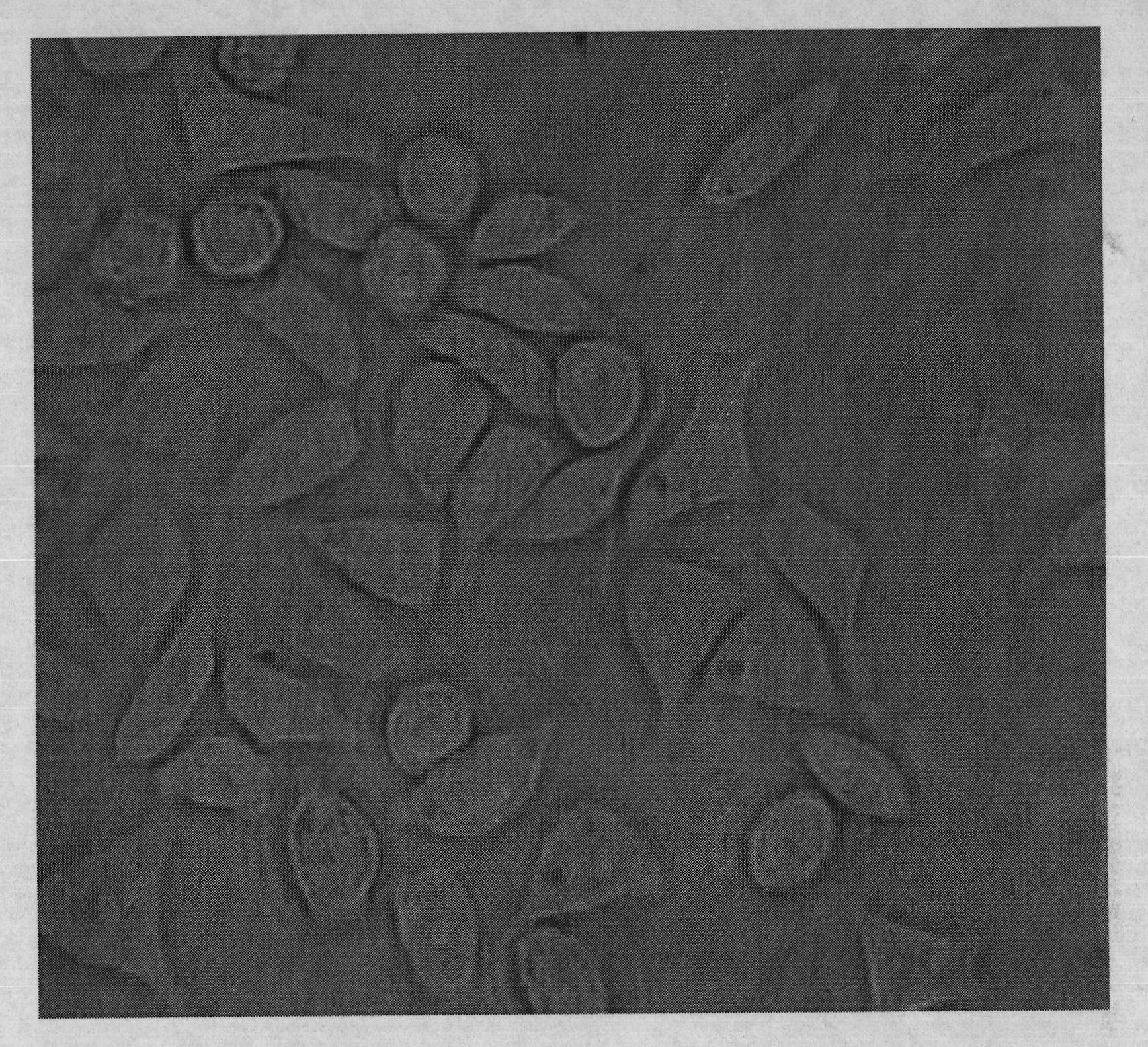
[0066] Cut the cross-linked hyaluronic acid material prepared in Example 1 or 2 into rectangular strips with a size of 12mm×3.0mm×2.0mm, and sterilize with ethylene oxide for future use. The experiment used big-eared white rabbits with a body weight of 2-3KG and was anesthetized by intramuscular injection of 50.0 mg of ketamine. The eyelid was opened with the eyelid speculum, and the bulbar conjunctiva of the fornix, which was 7.0 mm away from the corneoscleral limbus, was cut with ophthalmic micro scissors, and the length was about 10 mm. Separate the superior rectus muscle and oblique muscle, and use 5-0 silk thread to make two mattress sutures with a distance of 7.0 mm on the sclera at a distance of 4.0 mm from the corneoscleral limbus at both ends of the muscle. The prepared material was placed under the muscle and mattress suture, the suture was ligated, both ends of the material were trimmed, and the bulbar conjunctiva was sutured continuously with 5-0 silk thread. B-ul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transmittivity | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com