Saponin compound, preparation method for same, and application thereof in immunologic adjuvant preparation
An immune adjuvant and compound technology, applied in the field of saponin compounds and their preparation, can solve the problems of crystal precipitation, high salt content, and insufficient antigen effectiveness after long-term storage, and achieve great clinical application value, simple preparation method, high quality easily controlled effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Preparation of Saponin Compounds
[0049] (1) Dried root of Jintiesuo (commercially available, place of origin, Nujiang, Yunnan) (41kg), crushed and extracted three times under reflux with 80% ethanol (150L) (extraction intervals were 4 hours, 3 hours, 3 hours), combined extracts, and reduced Concentrate under pressure to obtain extract (no alcohol smell), after 0.86kg of extract is diluted with 8.6L of pure water, pass through a macroporous resin column (HP-20), use pure water (400L), 70% ethanol (300L) respectively, Acetone (200 L) eluted sequentially. The 70% ethanol elution part is mainly the total saponin, and the total saponin part is passed through a C-18 reversed-phase bonded silica gel column (ODS), and then eluted with pure water, 30% ethanol, 70% ethanol, and 90% ethanol in sequence (5 L each), divided into four elution fractions (S1-S4). Segment S4 was passed through a silica gel column and eluted with methanol-ethyl acetate 1:1 (the total amount...
Embodiment 2
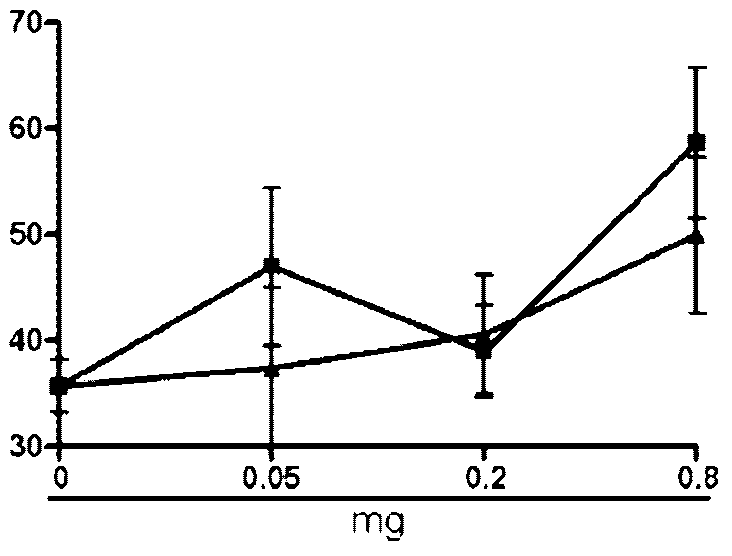
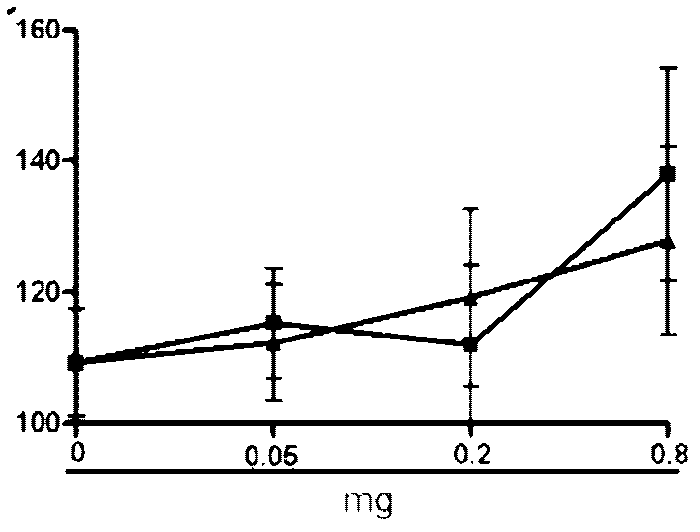
[0066] Example 2 Hemolytic Determination
[0067] Blood was collected from rabbit ear veins with a vacuum blood collection tube, added with normal saline, mixed evenly, centrifuged at 2000r for 10 min, washed with normal saline for 3 times, red blood cells were collected, and diluted with normal saline to make 0.5% red blood cell suspension. Take an appropriate amount of auroside A, B, and C prepared in Example 1, and dilute them with physiological saline to prepare a 4 mg / mL solution. After filtering through a 0.22 μL microporous membrane, it was diluted with normal saline to obtain dilutions with concentrations of 1000, 500, 250, 100, 50, 25, 10, and 5 μg / mL. In a 96-well plate, add 100 μL of 0.5% erythrocyte suspension to each well, then add 100 μL of saponin dilution solution of different concentrations, mix well, and repeat for 3 wells with one concentration. Then set the maximum and minimum hemolysis controls, which are physiological saline and distilled water respectiv...
Embodiment 3
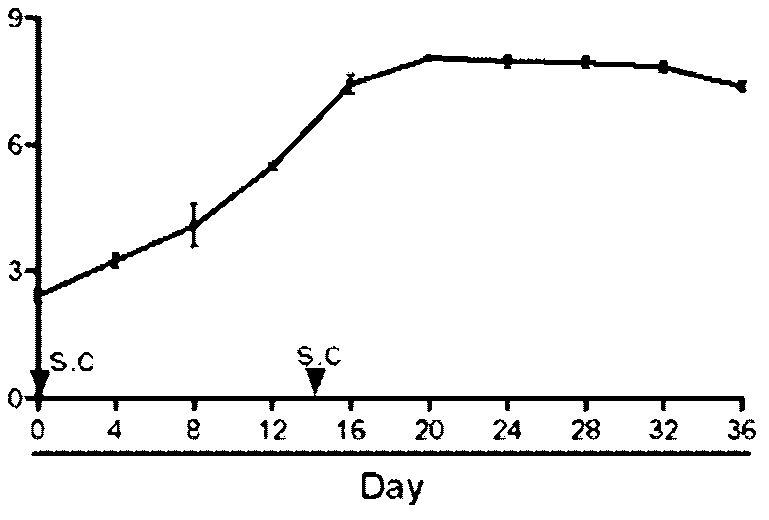
[0073] Example 3 Acute Toxicology
[0074] Dilute samples Quil A, aureoside B, and aureoside C with PBS to 5 mg / kg, 10 mg / kg, 20 mg / kg, 40 mg / kg, and 80 mg / kg respectively, 5 dose groups, and use PBS as a blank control group, There were 16 groups in total, and the ICR mice were randomly divided into 16 groups, with 5 mice in each group. The mice in each group were subcutaneously injected with the corresponding dose in the neck, and were reared for 72 days, and the toxic reactions (hair loss, pain, swelling, etc.) and death of the mice were observed.
[0075] The results of acute toxicology of auroside B and auroside C are shown in Table 2
[0076]
[0077]
[0078] As can be seen from Table 2-1, when Quil A was at 100 μg, 2 mice had died, showing strong toxicity, and the median lethal dose (LD 50 ) is 125 μg. And observed obvious toxic reactions within 72 hours, such as severe hair loss at the injection site, accompanied by pain and swelling, lethargy and loss of appe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com