A gastrodin compound and its pharmaceutical composition
A technology of gastrodin and its compounds, which is applied in the field of gastrodin compounds and their pharmaceutical compositions, can solve the problems of complicated process, unstable related substances, unstable pH value of gastrodin compounds, etc., achieve simple prescription process, improve safety and Effectiveness, stability improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
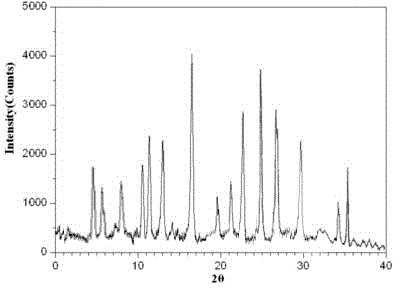
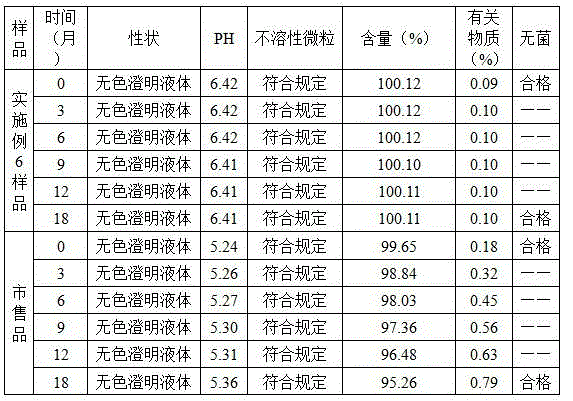
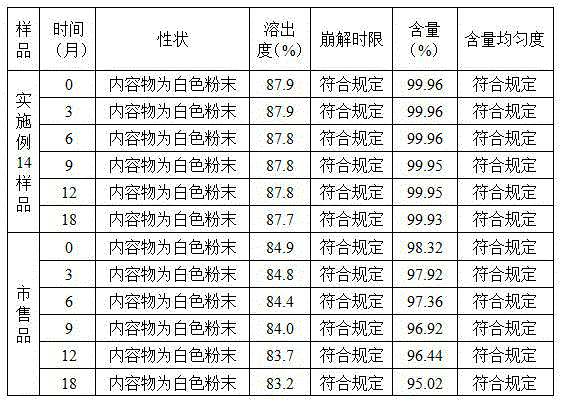
Image
Examples
Embodiment 1
[0050] Example 1 Preparation of gastrodin compound
[0051] Gastrodin (commercially available gastrodin raw material, Jiangsu Hanstone Pharmaceutical Co., Ltd.) was dissolved in ethanol and methanol with a volume ratio of 2:1 at a weight ratio of 1:8 at a stirring speed of 150 rpm at 20°C. In the mixed solution; keep at 20°C, add activated carbon with 1.0% gastrodin by weight, stir at 150 rpm for 25 minutes, filter to remove the carbon, filter the filtrate through a 0.22 μm filter membrane; 25°C, at a stirring speed of 100 rpm , while stirring at a speed of 40ml / min, slowly and uniformly dripped the mixed solution of chloroform and ether with a volume ratio of 4:1 whose weight is 7 times of the mixture of ethanol and methanol; , cooled to 2°C at a rate of 0.1°C / min for 10 hours, and filtered; the filter cake obtained by filtration was washed twice with a mixed solution of chloroform and ether with a volume ratio of 3 times the weight of 4:1, and dried. That is, the gastrodin ...
Embodiment 2
[0052] Example 2 Preparation of gastrodin compound
[0053] Gastrodin (commercially available gastrodin raw material, Jiangsu Hanstone Pharmaceutical Co., Ltd.) was dissolved in ethanol and methanol with a volume ratio of 2:1 at a weight ratio of 1:10 at a stirring speed of 200 rpm at 25°C. In the mixed solution; keep at 25°C, add activated carbon with 1.0% gastrodin by weight, stir at 200 rpm for 25 minutes, filter to remove the carbon, filter the filtrate through a 0.22 μm filter membrane; 30°C, at a stirring speed of 150 rpm , while stirring, slowly and uniformly dropwise at a speed of 50ml / min the mixed solution of chloroform and ether with a volume ratio of 4:1 that is 9 times the weight of the mixture of ethanol and methanol; , cooled to 4°C at a rate of 0.2°C / min for 10 hours, and filtered; the filter cake obtained by filtration in 4 was washed twice with a mixed solution of chloroform and ether with a volume ratio of 3 times the weight of 4:1. After drying, the gastro...
Embodiment 3
[0054] Example 3 Preparation of gastrodin lyophilized powder for injection (specification: 0.1g)
[0055] prescription:
[0056] The gastrodin compound prepared in Example 1 100g
[0057] Mannitol 60g
[0058] Water for injection 2000ml
[0059] ____________________________________
[0060] 1000 pieces
[0061] Craft:
[0062] 1. Add the prescribed amount of gastrodin to 70% of the water for injection, stir and dissolve at 30°C, add water for injection to 80%, then add mannitol according to the prescribed amount, stir and dissolve, add water for injection to the full amount, and stir well.
[0063] 2. Add 0.15% activated carbon to 1, stir for 20 minutes, filter and decarbonize, filter and sterilize with 0.22 μm filter membrane, and conduct intermediate detection.
[0064] 3. Freeze-dried:
[0065] ①Pre-freezing: divide the 2 filtrates into half plugs and place them in a freezer that is pre-cooled to -25°C, keep for 1 hour, cool down to -35°C at a speed of 0.2°C / min, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com