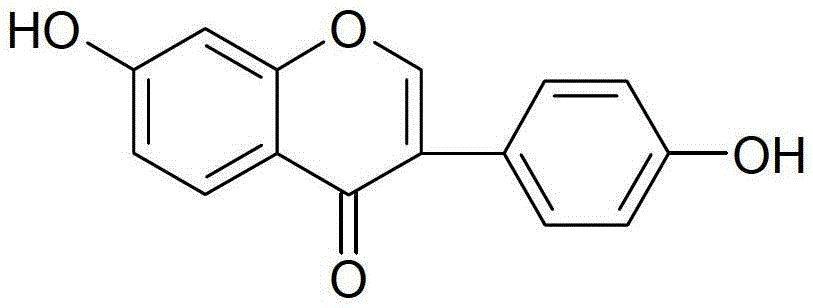
Daidzein-containing tablet composition and preparation method thereof
A technology of daidzein and aglycone tablets, which can be applied to medical preparations containing active ingredients, medical preparations without active ingredients, and drug combinations, etc., can solve the problem of affecting clinical efficacy and clinical application scope, and restricting the route of administration. and dosage forms, poor dissolution of oral preparations, etc., to achieve the effect of benefiting human absorption, improving dissolution, and ensuring drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
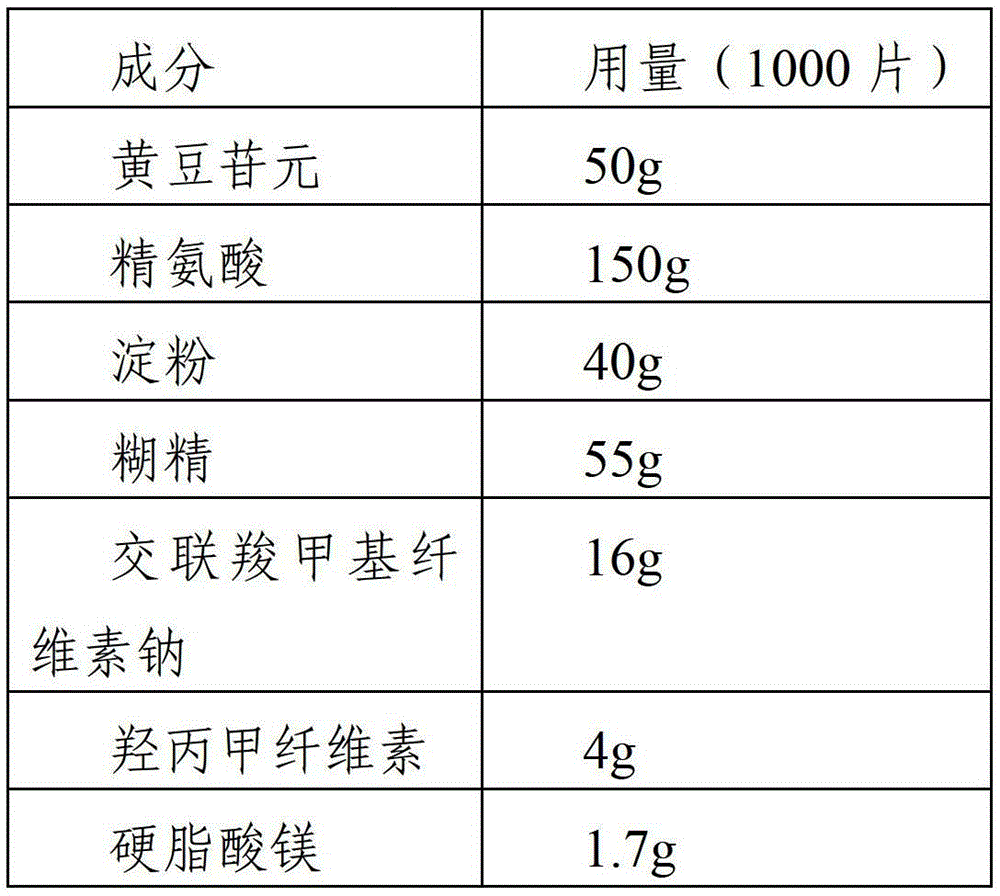
[0039] Example 1 Preparation of daidzein tablets
[0040]
[0041] The preparation process is as follows:
[0042] (1) Take daidzein, 35% arginine, and 20% disintegrating agent according to the prescription, mix well and make soft material with ethanol solution of 30% binder in the prescription amount, sieve with 20 meshes to make wet granules, 60 Dry at ℃ for 2h;
[0043] (2) Take the remaining arginine, the prescribed amount of diluent and 15% disintegrant, mix and add the ethanol solution of the remaining binder to make a soft material, sift through a 20-mesh sieve to make wet granules, and dry at 60°C for 2 hours;
[0044] (3) Mix the granules obtained in step 1 and step 2, add the remaining disintegrant and the prescribed amount of lubricant at the same time, mix well and press into tablets, the specification is 50mg / tablet.
Embodiment 2
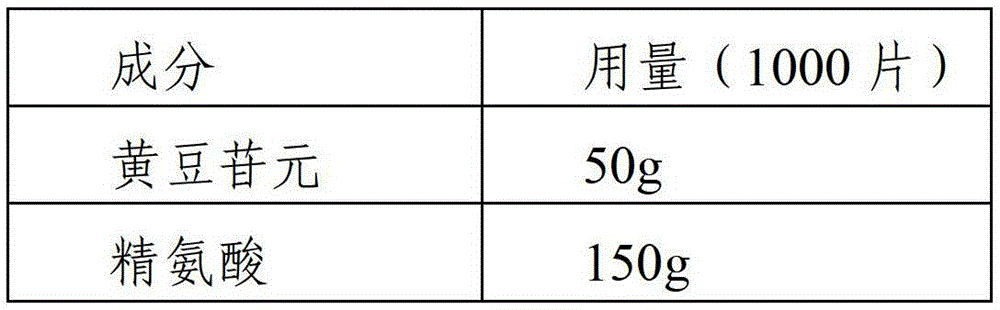
[0045] Example 2 Preparation of daidzein tablets
[0046]
[0047]
[0048] The preparation process is as follows:
[0049] (1) Take daidzein, 30% arginine, and 10% disintegrant according to the prescription, mix well and make a soft material with an ethanol solution of 25% binder in the prescription amount, sieve through a 20-mesh sieve to make wet granules, 60 Dry at ℃ for 2h;
[0050] (2) Take the remaining arginine, the diluent of the prescribed amount and 20% of the disintegrating agent, mix and add the ethanol solution of the remaining binder to make a soft material, sieve through 20 meshes to make wet granules, and dry at 60°C for 2 hours;
[0051] (3) Mix the granules obtained in step 1 and step 2, add the remaining disintegrant and the prescribed amount of lubricant at the same time, mix well and press into tablets, the specification is 50mg / tablet.
Embodiment 3
[0052] Example 3 Preparation of daidzein tablets
[0053]
[0054] The preparation process is as follows:
[0055] (1) Take daidzein, 35% arginine, and 20% disintegrant according to the prescription, mix well, make soft material with ethanol solution of 30% binder in the prescription amount, and sieve through 20 meshes to make wet granules. Spread the wet granules on an enamel plate, put them in a blower constant temperature drying oven, and dry them at 60°C for 2 hours;
[0056](2) Take the remaining arginine, the prescribed amount of diluent and 18% disintegrant, mix and add the ethanol solution of the remaining binder to make a soft material, and sieve through a 20-mesh sieve to make wet granules. Spread the wet granules on an enamel plate, put them in a blower constant temperature drying oven, and dry them at 60°C for 2 hours;
[0057] (3) Mix the granules obtained in step 1 and step 2, add the remaining disintegrant and the prescribed amount of lubricant at the same ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com