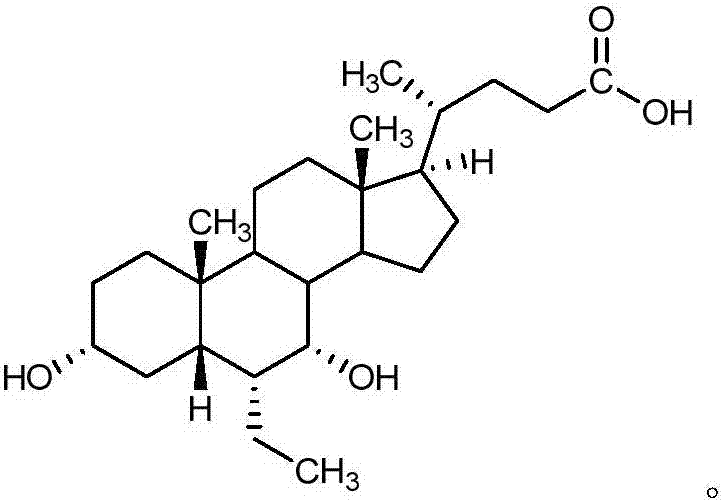
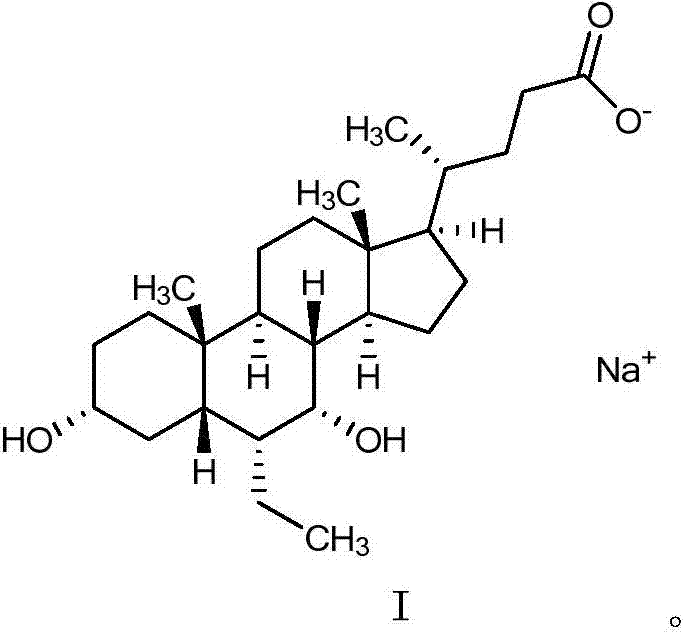
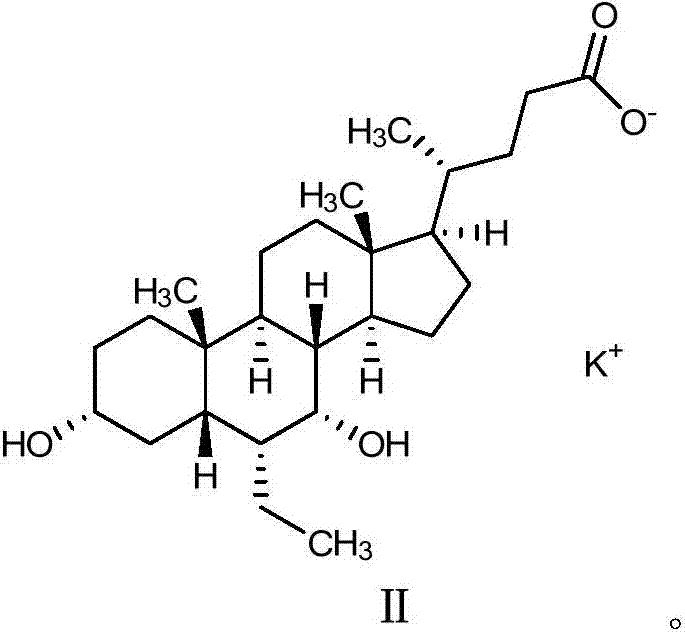
Obeticholic acid salts and pharmaceutical composition thereof
A technology of obeticholic acid salt and obeticholic acid potassium salt, which is applied in the field of medicinal chemistry and can solve problems such as poor stability of obeticholic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Embodiment 1 obeticholic acid sodium salt
[0081] Preparation method one
[0082] Add obeticholic acid (2.0 g, 4.75 mmol) into methanol (20.0 mL, 10 times the amount v / w), and stir until dissolved; add purified water (4 mL, 2 times the amount v / w) and sodium hydroxide ( 0.19g, 4.75mmol); stirred at 10-30°C for 1h, concentrated to dryness under reduced pressure at 40°C to obtain an oily substance; added acetonitrile (20.0mL, 10 times the amount v / w) into the reaction flask containing the above solid, stirred After dispersion, beat for 1 hour; filter with suction, wash the filter cake once with acetonitrile (4.0 mL, 2 times the amount v / w), and dry under reduced pressure at 65-75°C for 8 hours. 2.0 g of white solid was obtained, yield: 95%.
[0083] Preparation method two
[0084] Sodium hydroxide (0.38g, 9.51mmol) was added to purified water (20mL, 5 times the amount v / w), and obeticholic acid (4.0g, 9.51mmol) was slowly added, stirred until dissolved, and the insolu...
Embodiment 2
[0086] Embodiment 2 obeticholic acid potassium salt
[0087]Add obeticholic acid (2.0 g, 4.75 mmol) into methanol (20.0 mL, 10 times the amount v / w), and stir until dissolved; add purified water (4 mL, 2 times the amount v / w) and potassium hydroxide ( 0.27g, 4.75mmol); Stir at 10~30°C for 1h, concentrate to dryness under reduced pressure at 40°C to obtain foam or oil; add acetonitrile (20.0mL, 10 times the amount v / w) into the reaction flask containing the above solid , after stirring and dispersing, beat for 1 h; filter with suction, wash the filter cake once with acetonitrile (4.0 mL, 2 times the amount v / w), and dry under reduced pressure at 65-75°C for 8 h. 2.04 g of white solid was obtained, yield: 94%.
[0088] Elemental analysis: Ret.Time=6.11min, Height=74.09μS, Area=17.61μS*min.
Embodiment 3
[0089] Embodiment 3 Obeticholic acid magnesium salt
[0090] Preparation method one
[0091] Add sodium hydroxide (0.19g, 4.75mmol) into purified water (20mL, 10 times the amount v / w), slowly add obeticholic acid (2.0g, 4.75mmol), stir until dissolved, and filter out insoluble matter; Magnesium sulfate heptahydrate (0.58g, 2.38mmol) was added to purified water (5mL, 2.5 times the amount v / w), stirred until dissolved; the magnesium salt solution was dropped into the obeticholic acid sodium salt solution, and stirred after the dropping was completed After 1h, filter with suction, wash the filter cake twice with purified water (20mL×2, 10 times the amount v / w), and dry under reduced pressure at 65-75°C for 8h. 1.96 g of white solid was obtained, yield: 95%.
[0092] Preparation method two
[0093] Add triethylamine (0.66mL, 4.75mmol), acetone (2mL, 1 times v / w), purified water (8mL, 4 times v / w) into the reaction flask, slowly add obeticholic acid (2.0g , 4.75mmol), stirred u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com