Bromhexine hydrochloride compound and pharmaceutical composition thereof
A technology of bromhexine hydrochloride and a compound, applied in the field of medicine, can solve the problems of high process requirements, instability of bromhexine hydrochloride, poor stability, etc., and achieves the advantages of simple prescription process, improved safety and effectiveness, and improved stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The preparation of embodiment 1 bromhexine hydrochloride compound
[0052] Put 100g of bromhexine hydrochloride crude product in a mixed solution of 1200g ethanol and methanol (volume ratio 15:1), heat up to 50°C at 0.5°C / min while stirring at a speed of 150 rpm, and stir until completely dissolved; Maintain 50°C, add 1.5g of activated carbon, stir at 200 rpm for 30 minutes, filter to remove carbon, and filter the filtrate through a 0.22μm filter membrane; at 45°C, at a stirring speed of 100 rpm, stir while stirring Add 4800g of a mixed solution of water and acetone with a volume ratio of 5:1 dropwise at a speed of 1 min; cool down to 8°C at 2°C / min, stop stirring; cool down to 2°C at 0.2°C / min and let stand to grow crystals for 12 hours, filter; filter the cake Wash twice with 2 times the weight of acetone, and dry in vacuum at 45° C. for 8 hours to obtain the bromhexine hydrochloride compound.
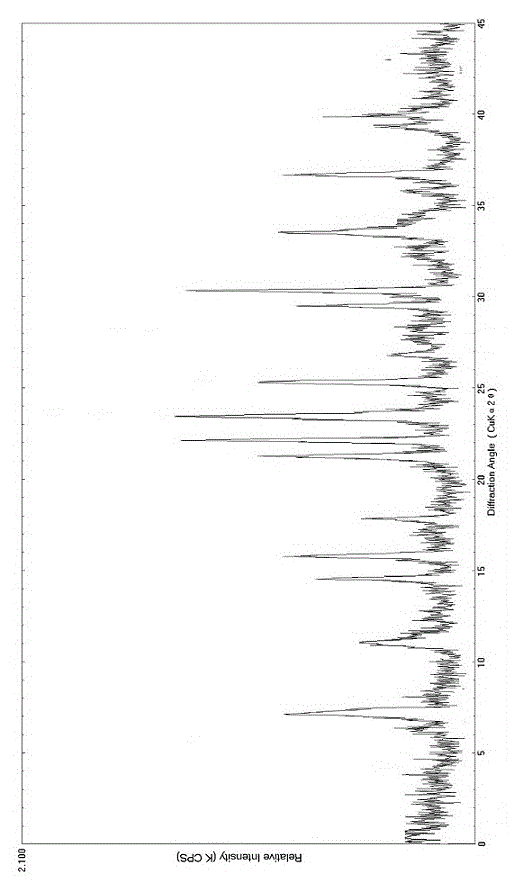
[0053] The melting point of this compound is 245-248°C. X-ray powder di...
Embodiment 2
[0054] Embodiment 2 The preparation of bromhexine hydrochloride compound
[0055] Put 100 g of bromhexine hydrochloride crude product in a mixed solution of 1200 g ethanol and methanol (volume ratio 15:1), heat up to 50 °C at 0.5 °C / min while stirring at a speed of 200 rpm, and stir until completely dissolved; Maintain 50°C, add 1.5g of activated carbon, stir at 250 rpm for 30 minutes, filter to remove carbon, and filter the filtrate through a 0.22μm filter membrane; Add 4800g of a mixed solution of water and acetone with a volume ratio of 5:1 dropwise at a speed of 1 min; cool down to 12°C at 2.5°C / min, stop stirring; cool down to 5°C at 0.5°C / min and let the crystal grow for 12 hours, filter; filter the cake Wash twice with 2 times the weight of acetone, and dry in vacuum at 50° C. for 10 hours to obtain the bromhexine hydrochloride compound. The melting point of this compound is 245-248°C. The X-ray powder diffraction pattern is consistent with Example 1.
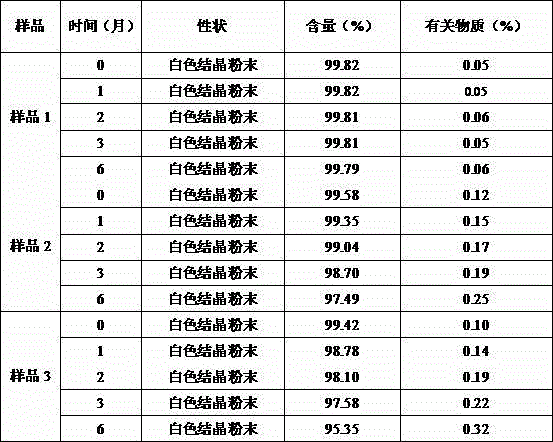
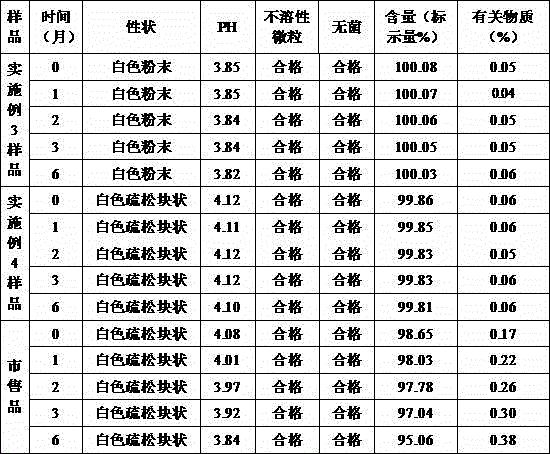
[0056] The br...
Embodiment 3
[0057] The preparation of embodiment 3 bromhexine hydrochloride compound powder injection (specification: 4mg)
[0058] The bromhexine hydrochloride compound prepared by the present invention is aseptically sub-packed according to the bromhexine hydrochloride dose of 4 mg / bottle in a 100-grade aseptic environment, stoppered, and packed after passing the crimping inspection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com