A stable busulfan injection
A busulfan injection and a technology for the busulfan injection, which are applied in the field of busulfan injection, can solve the problems of reducing drug efficacy, drug waste, increasing costs, etc., and achieve the effects of safety in the preparation process, improving stability and ensuring safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
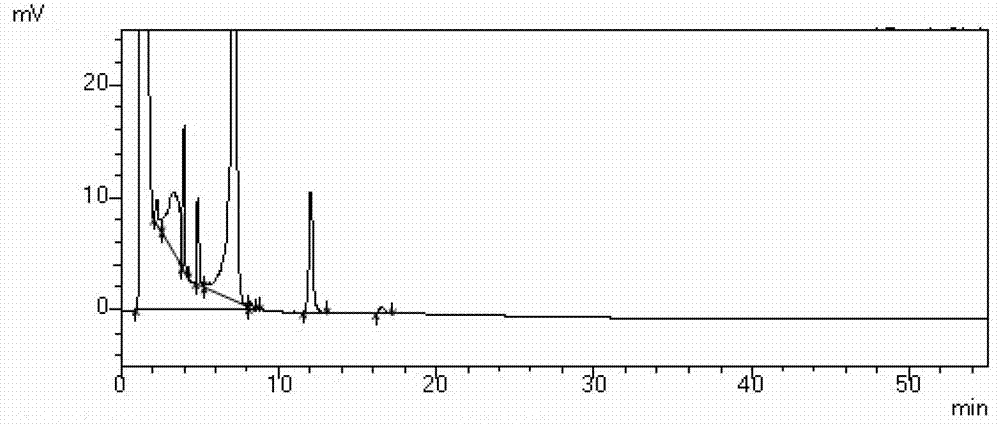
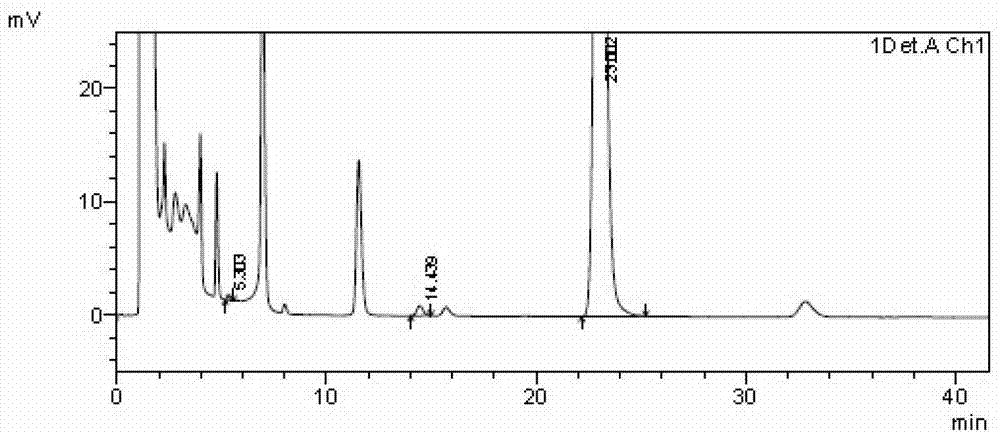
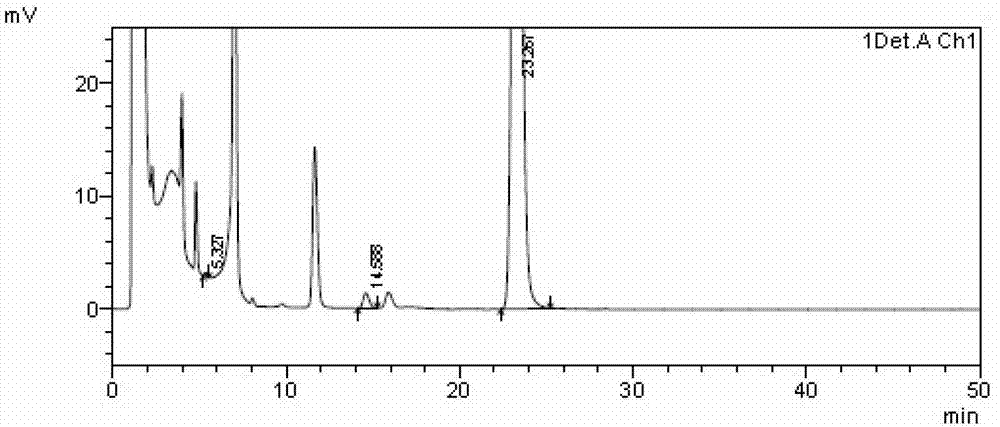
Image
Examples
Embodiment 1
[0043] (1) Take 60mg of busulfan, and dissolve busulfan in 10ml of a mixed solvent of N,N-dimethylacetamide and polyethylene glycol 400 with a volume ratio of 33:67 at a controlled temperature of 20°C; (2 ) was filtered through a 0.45 μm microporous membrane, and the filtrate was filtered through a 0.22 μm microporous membrane for aseptic filling to obtain busulfan injection.
[0044] In the preparation method of the present invention, the humidity control of the working environment refers to the prior art.
Embodiment 2
[0046] (1) Take 60mg of busulfan, and dissolve busulfan in 10ml of a mixed solvent of N,N-dimethylacetamide and polyethylene glycol 400 with a volume ratio of 33:67 at a controlled temperature of 16°C; (2 ) with a 0.45 μm microporous membrane filter. Take the filtrate, filter it with a 0.22 μm microporous membrane and fill it aseptically to obtain busulfan injection.
Embodiment 3
[0048] Take N,N-dimethylacetamide and polyethylene glycol 400 (the volume ratio of the two solvents is shown in Table 1), mix well, and then fill the mixed solvent with nitrogen to achieve saturation (in order to ensure that the nitrogen is saturated, it may appear After continuous uniform air bubbles, continue to fill with nitrogen for 5 minutes). Add 60 mg of busulfan raw material to the mixed solvent, stir to dissolve, control the temperature at 16° C. during the dissolution process, and filter with a 0.45 μm microporous membrane. Take the filtrate, filter it with a 0.22 μm microporous membrane, and then continue to fill the mixed solvent with nitrogen to achieve saturation until continuous and uniform bubbles appear, and this state lasts for 5 minutes. Aseptic filling.
[0049] Table 1 prepares the solvent ratio and consumption selection of busulfan injection
[0050]
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com