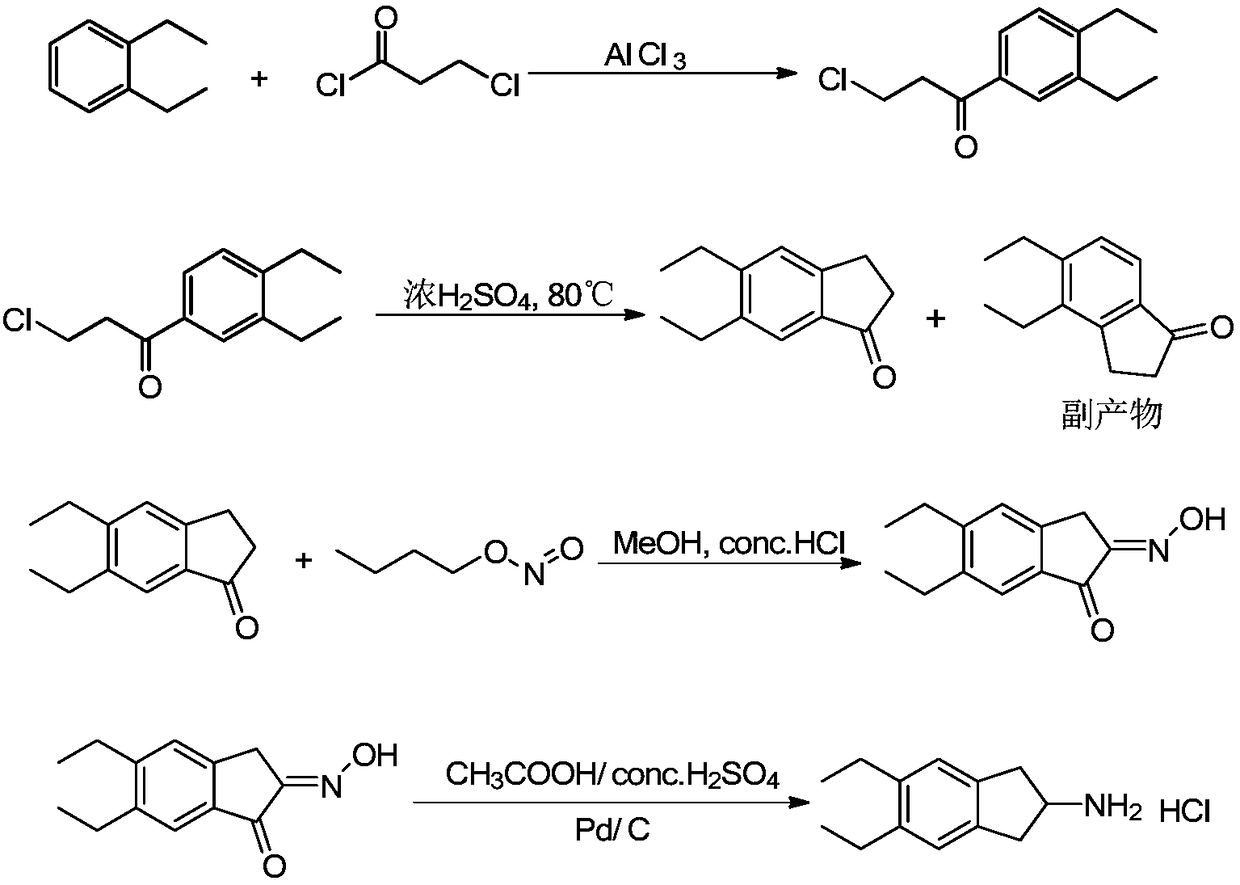
Preparation method of indacaterol intermediate
A technology of intermediates and compounds, applied in the field of preparation of pharmaceutical intermediates, can solve the problems of high price, difficult industrial production, poor economy and environmental friendliness, and achieve the effect of good selectivity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
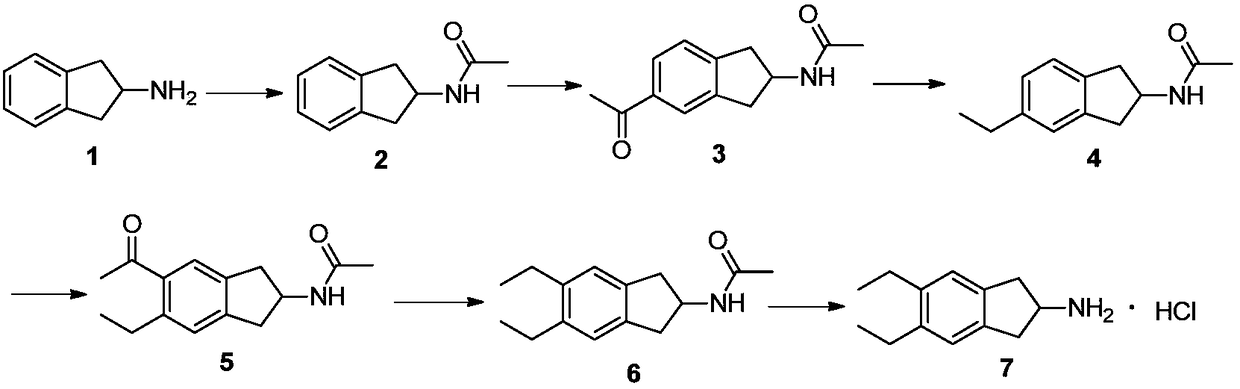
[0026] Embodiment 1, preparation of indacaterol intermediate 5,6-diethyl-2,3-dihydro-1H-indene-2-amine hydrochloride
[0027]
[0028] (1) Synthesis of N-(2,3-dihydro-1H-inden-2-yl)acetamide (compound 2)
[0029] Add compound 1 (100g, 0.751mol) and dichloromethane (700ml) into a 2L three-necked flask, then add triethylamine (189.6g, 1.877mol), control the temperature below 10°C, add acetyl chloride (64.9g, 0.827mol), after the addition, the temperature was raised to room temperature for 2 hours, and TLC monitored the reaction to be complete. Add 2N hydrochloric acid (200ml) to quench the reaction, separate the layers, wash the organic phase with tap water (150ml×2), and dry over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure to obtain 126.8 g of white solid (compound 2), with a yield of 96.3%.
[0030] (2) Synthesis of N-(5-acetyl-2,3-dihydro-1H-inden-2-yl)acetamide (compound 3)
[0031] Add aluminum trichloride (106.7g, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com