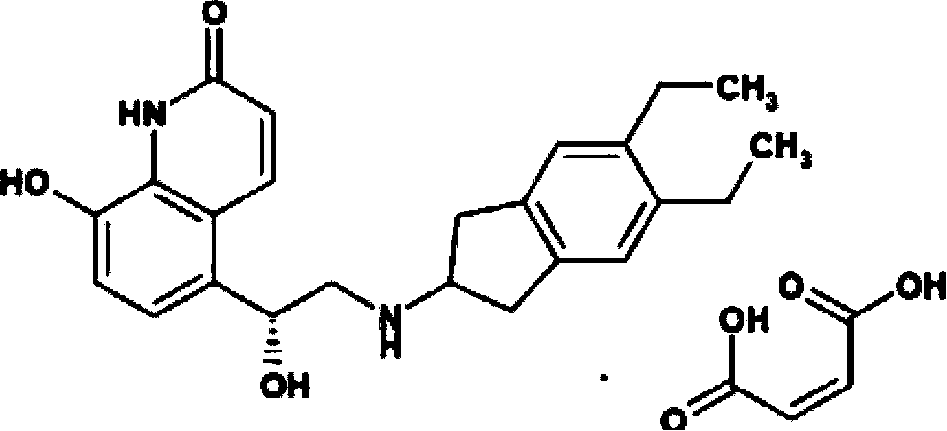
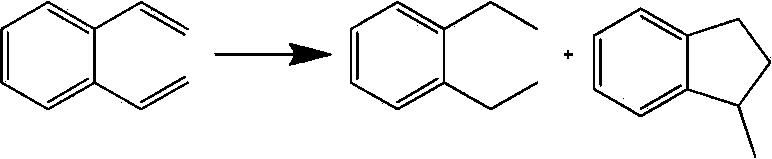
Preparation method of indacaterol intermediate diethylbenzene
A technology for o-diethylbenzene and o-ethylacetophenone, which is applied in the field of preparing o-diethylbenzene and can solve problems such as large environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] o-Ethylacetophenone (29.6g, 0.20mol), 80% hydrazine hydrate (97.7ml, 1.6mol) and absolute ethanol (400ml) were mixed, magnetically stirred, and refluxed at 25°C for 2-6 hours. After the reaction, add appropriate amount of distilled water, extract with ethyl acetate, wash with saturated brine, anhydrous Na 2 SO 4 Drying and rotary evaporation gave white crystal II (28.9 g, yield 89.1%). 1H-NMR (CDCl3) δ7.24(d, 2H), 7.16(t, 2H), 5.44(s, 2H), 2.68(m, 2H), 2.15(s, 3H), 1.23(t, 3H).
Embodiment 2
[0024] o-Ethylacetophenone (45.00g, 0.30mol), 80% hydrazine hydrate (18.3ml, 0.30mol) and absolute ethanol (700ml) were mixed, stirred by magnetic force, and reacted at 25°C for 8 hours. After the reaction, add appropriate amount of distilled water, extract with ethyl acetate, wash with saturated brine, anhydrous Na 2 SO 4 Drying and rotary evaporation gave white crystal II (40.1 g, yield 82.4%).
Embodiment 3
[0026] o-Ethylacetophenone (29.6g, 0.20mol), 80% hydrazine hydrate (244ml, 4mol) and absolute ethanol (400ml) were mixed, stirred by magnetic force, and reacted at 100°C for 3 hours. After the reaction, add appropriate amount of distilled water, extract with ethyl acetate, wash with saturated brine, anhydrous Na 2 SO 4 Drying and rotary evaporation gave white crystal II (27.7 g, yield 85.4%).
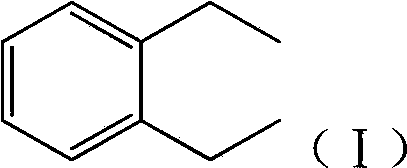
[0027] Compound formula (I)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com