Application of indacaterol maleate as cGAS-STING pathway targeted agonist
An agonist, acid indene technology, applied in the field of medicine, can solve the problems such as no article, no patent application, etc., and achieve the effects of high safety, low cost and good development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In order to achieve the above object, the present invention adopts the following technical solutions:
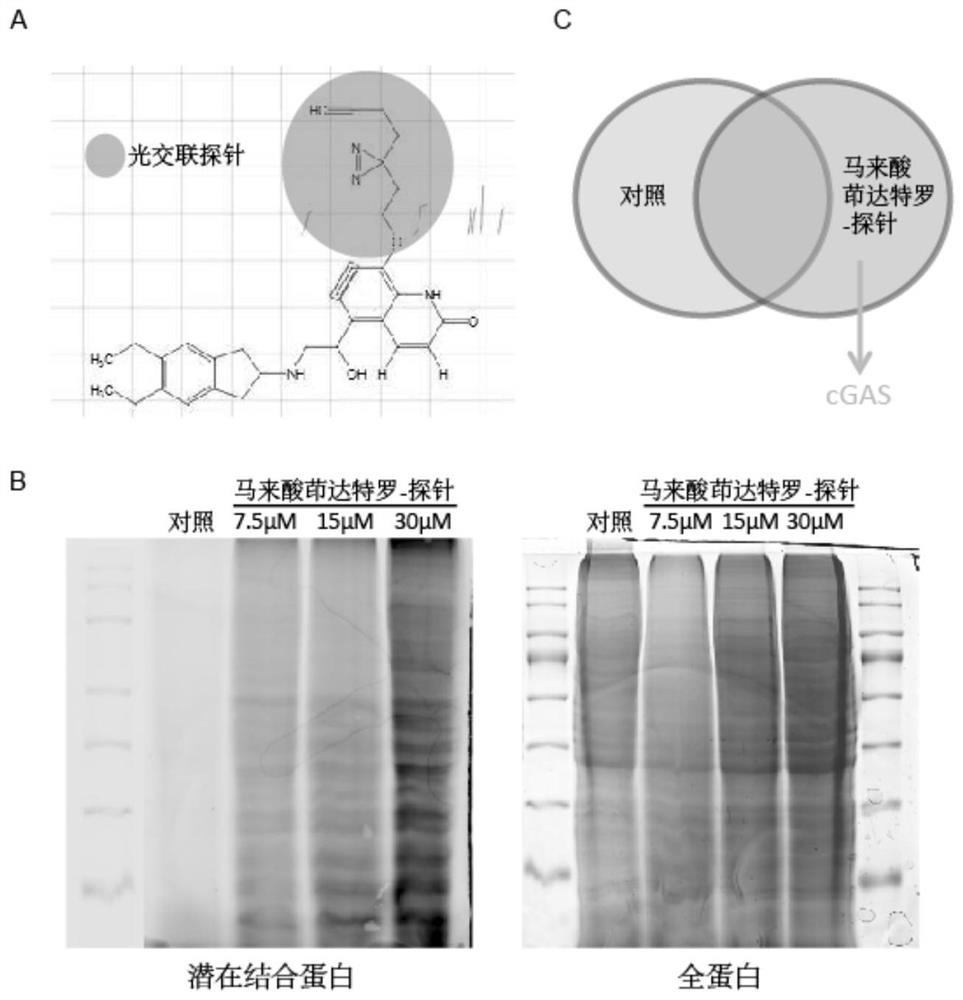
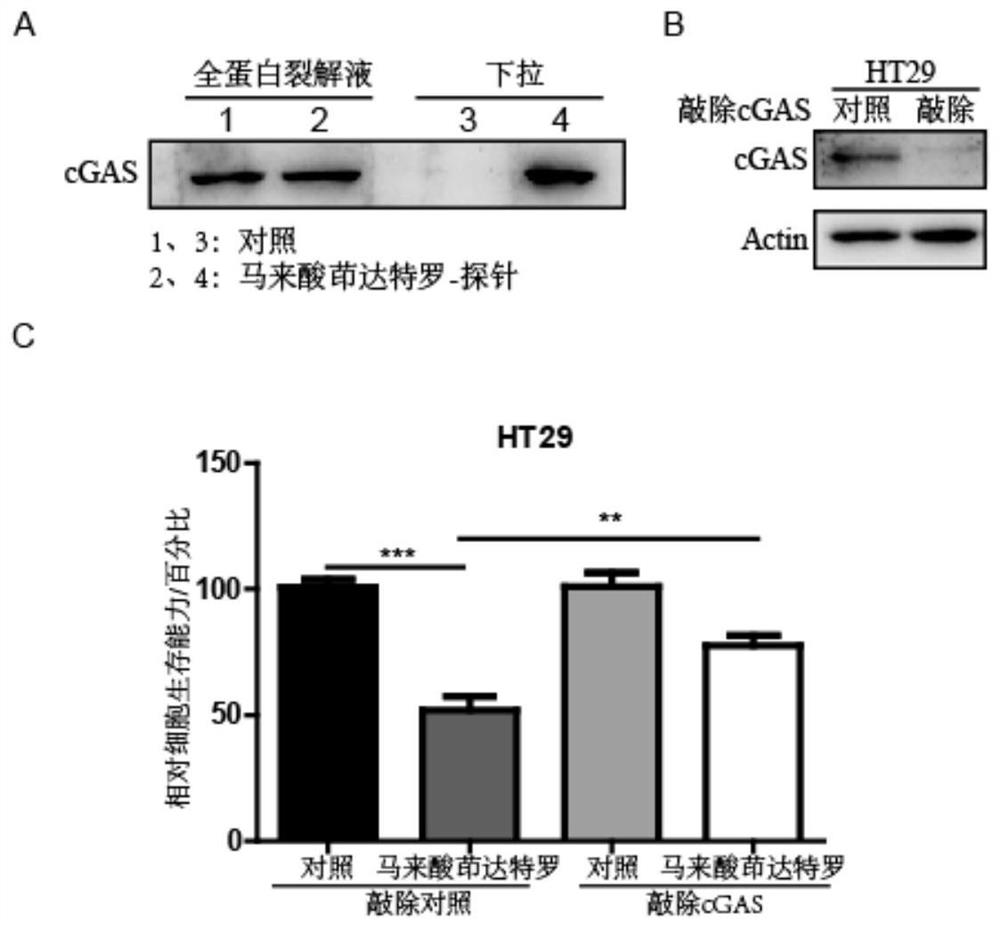
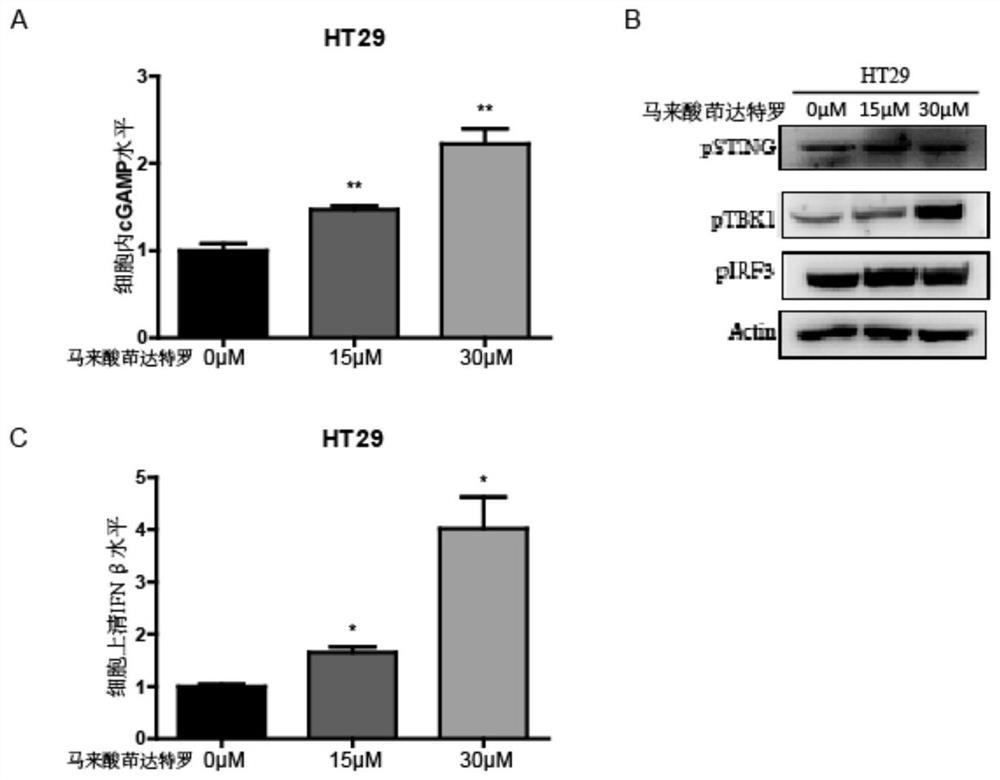
[0020] 1. Identification of indacaterol maleate targeting cGAS-STING pathway by biotin photoaffinity labeling combined with proteomics. The specific steps are:
[0021] (1) Combining indacaterol maleate with a photocrosslinking probe. Dissolve 50 mg of indacaterol maleate (purchased from Shanghai Taosu Biochemical Technology Co., Ltd.) in 5 mL of dimethylformamide (N,N-Dimethylformamide, DMF), and add 25.8 mg of probe diacridine (3 -(but-3-yn-1-yl)-3-(2-iodoethyl)-3H-diazirine) (purchased from Shanghai Bi De Pharmaceutical Technology Co., Ltd.) and 26 mg potassium carbonate, mix the solution and stir at 60 ° C 12 hours. After the reaction, 10 mL of ultrapure water was added to cool down, and extracted with ethyl acetate (twice, 10 mL each), combined with 10 mL of brine, washed the organic layer twice, and dried over anhydrous sodium sulfate. The residue remaining ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com