Preparation method of indacaterol and salt thereof
A technology for indacaterol salts and compounds, which is applied in the direction of carboxylate preparation, carboxylate preparation, organic compound preparation, etc., can solve the problems of high cost, low yield, low purity, etc., and achieve low cost and high yield. High rate, high quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
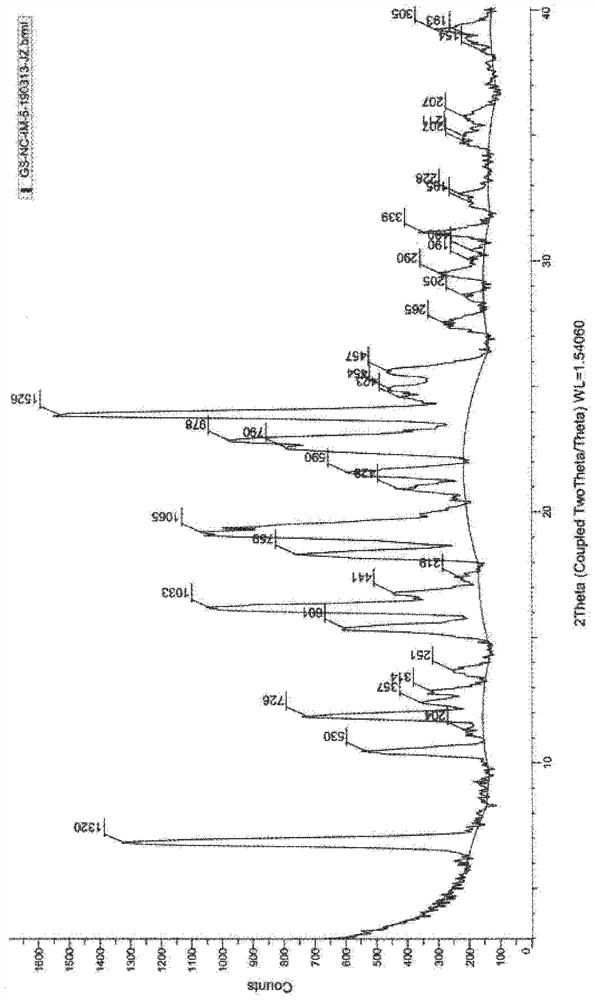
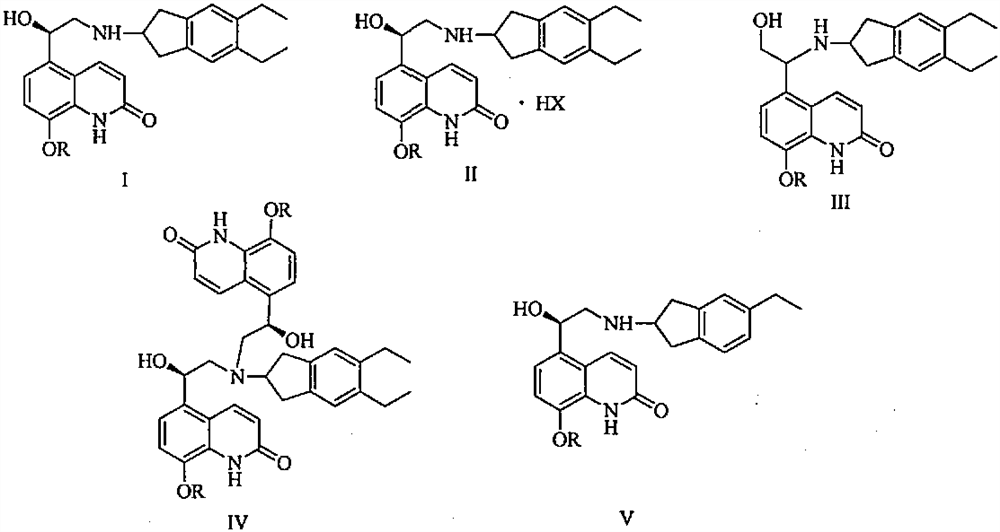
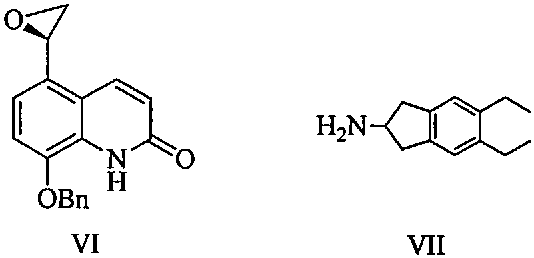
[0050] Add 2-amino-5,6-diethylindane (22.7g) and n-butanol 600ml into a 2L reaction flask, stir to dissolve, then add 8-phenylmethoxy-5-(R) -Oxiranyl-1H-quinolin-2-one (29.3 g, S isomer content 0.5%), heated to 110° C., stirred for 4 hours, TLC detected that the reaction was complete. Under reduced pressure, the reaction solution was concentrated to obtain a crude brown oily product, which was added with 800ml of ethanol and stirred to dissolve, and 200ml of ethanol solution of m-chlorobenzoic acid (31.3g) was added, stirred and dissolved at 80°C, cooled to 30°C, and stirred for 6 hours, filtered, washed twice with a small amount of ethanol, and dried at 60°C for 8 hours to obtain off-white solid product (R)-5-[2-(5,6-diethyl-indan-2-ylamino)-1 -Hydroxy-ethyl]-8-benzyloxy-2-ketone m-chlorobenzoate (47.9g), the yield is 75.1%, the HPLC purity is 99.8% (area normalized method), and the single impurity content is less than 0.05 %, S isomer content is 0.06%. HPLC impurity analys...
Embodiment 2
[0056] Add 2-amino-5,6-diethylindane (27.2g) and n-butanol 600ml into a 2L reaction flask, stir to dissolve, then add 8-phenylmethoxy-5-(R) to the solution -Oxiranyl-1H-quinolin-2-one (29.3g, S isomer content 0.5%), heated to 80°C, stirred for 8 hours, TLC detected that the reaction was complete. Under reduced pressure, the reaction solution was concentrated to obtain a crude brown oil, which was added with 400 ml of tetrahydrofuran and stirred to dissolve, then added with 200 ml of tetrahydrofuran solution of m-chlorobenzoic acid (78.2 g), stirred and dissolved at 50°C, cooled to 30°C, and stirred for 6 hours, filtered, washed twice with a small amount of tetrahydrofuran, and dried at 60°C for 8 hours to obtain an off-white solid product (R)-5-[2-(5,6-diethyl-indan-2-ylamino)-1 -Hydroxy-ethyl]-8-benzyloxy-2-ketone m-chlorobenzoate (46.3g), the yield is 72.5%, the HPLC purity is 99.8% (area normalized method), and the single impurity content is less than 0.05 %, S isomer cont...
Embodiment 3
[0058] Add 2-amino-5,6-diethylindane (20.8g) and n-butanol 600ml into a 2L reaction flask, stir to dissolve, then add 8-phenylmethoxy-5-(R) -Oxiranyl-1H-quinolin-2-one (29.3 g, S isomer content 0.5%), heated to 120° C., stirred for 6 hours, TLC detected that the reaction was complete. Under reduced pressure, the reaction solution was concentrated to obtain a crude brown oily product, which was added with 800ml of isopropanol and stirred to dissolve, then added with 200ml of isopropanol solution of m-chlorobenzoic acid (15.7g), stirred and dissolved at 80°C, and cooled to 30°C. ℃, stirred for 6 hours, filtered, washed twice with a small amount of isopropanol, and dried at 60°C for 8 hours to obtain the off-white solid product (R)-5-[2-(5,6-diethyl-indane-2 -ylamino)-1-hydroxyl-ethyl]-8-benzyloxy-2-ketone m-chlorobenzoate (48.2g), yield 75.5%, HPLC purity 99.7% (area normalization method) , the single hetero content is less than 0.05%, and the S isomer content is 0.08%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com