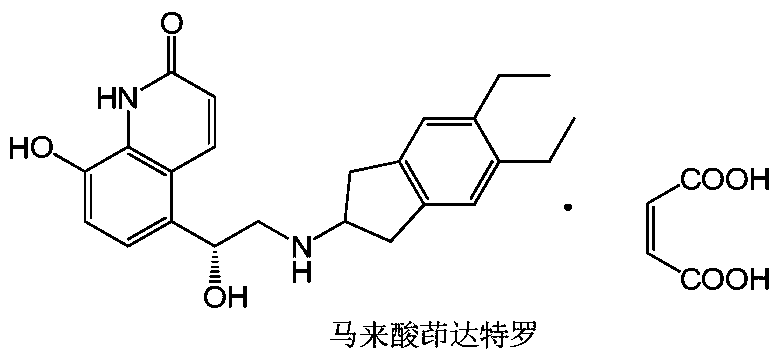
Improved preparation process of indacaterol maleate
A preparation process and acid indene technology, applied in the field of pharmaceutical synthesis, can solve the problems of difficulty in purifying intermediate 4, long reaction time, low efficiency, etc., and achieve the effects of shortened reaction time, high yield and purity, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
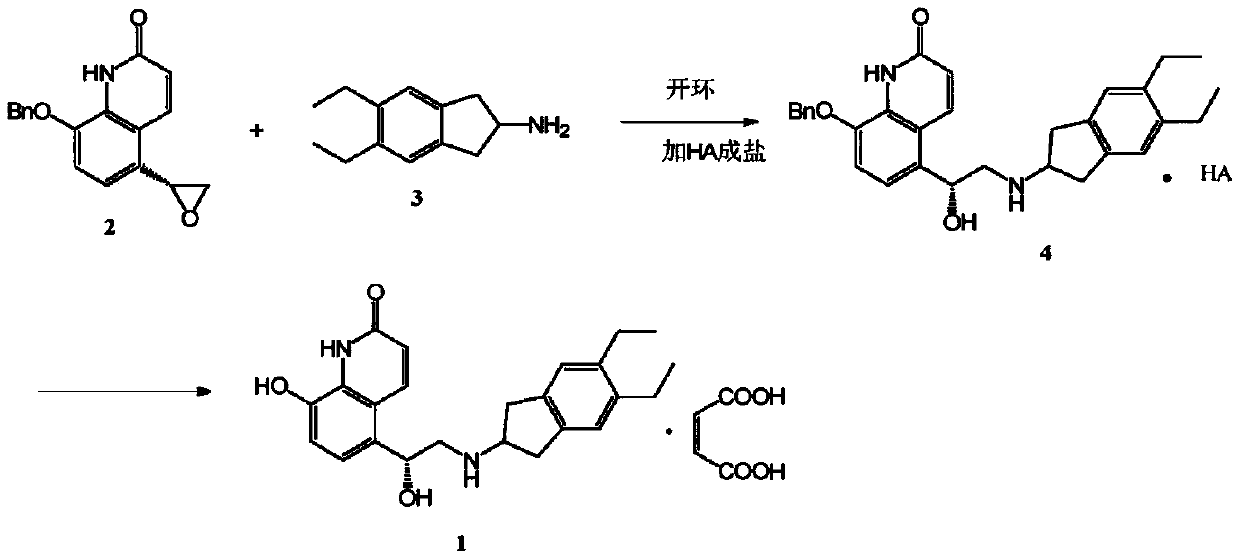
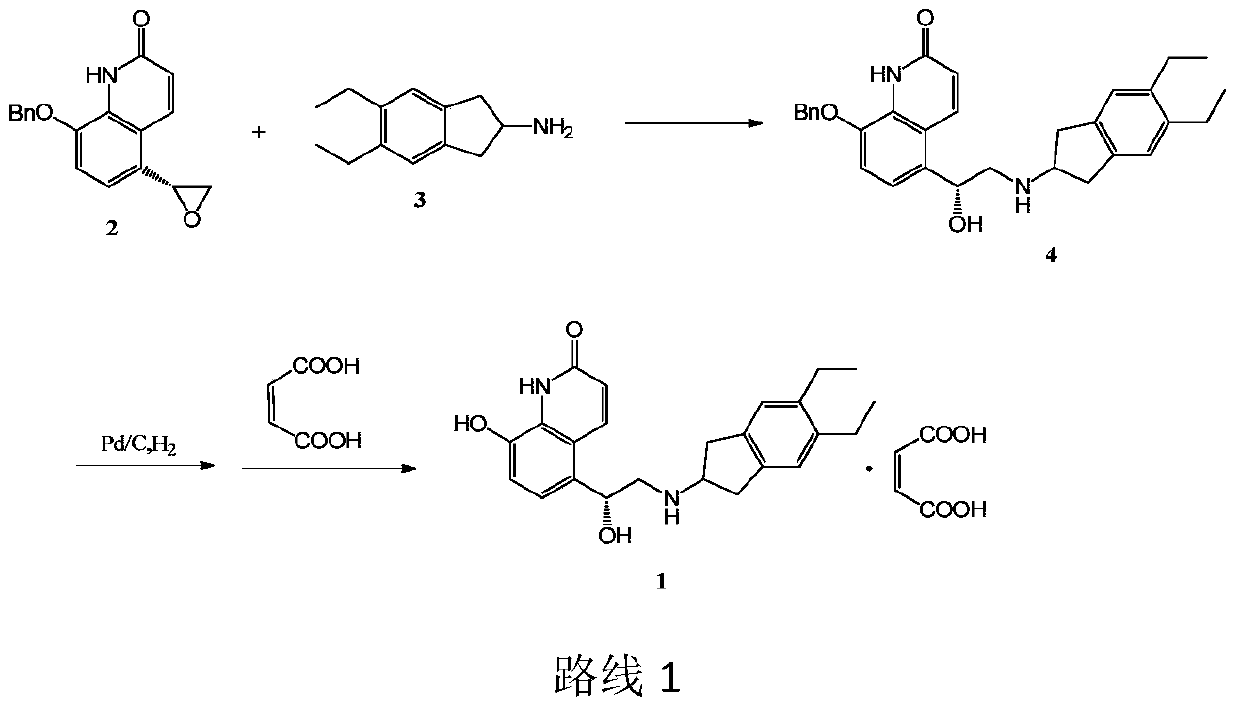
[0026] Example 1.5-[(R)-2-(5,6-diethyl-indan-2-ylamino)-1-hydroxy-ethyl]-8-phenylmethoxy-(1H)-quinone Preparation of lin-2-one p-methoxybenzoate
[0027]
[0028] Add 5.0 g of 5,6-diethyl-2,3-dihydro-1H-indene-2-amine and 7 mL of dimethylmethylene sulfone, 7.0 g of 5‐(2R)‐2‐oxiranyl‐8‐benzyloxy‐2(1H)‐quinolinone was added to the solution, the resulting suspension was heated to 110 °C, and Stirring at this temperature for 7h, TLC showed that the reaction was complete. Cool the resulting brown solution to 60°C, add 80mL of ethanol, keep at 60°C, then add 4.4g of p-methoxybenzoic acid to dissolve completely, cool the solution to about 40°C, add seed crystals, and place in an ice bath to cool. 11.4 g of crude p-methoxybenzoate were isolated by filtration and recrystallized from ethanol to give 10.6 g of 5‐[(R)‐2‐(5,6‐diethyl‐indan‐2‐ylamino)‐1‐ The pure product of hydroxy‐ethyl]‐8‐phenylmethoxy‐(1H)‐quinolin‐2‐one p-methoxybenzoate is a white crystalline powder (yield 70.1%,...
Embodiment 25
[0029] Example 2.5-[(R)-2-(5,6-diethyl-indan-2-ylamino)-1-hydroxy-ethyl]-8-phenylmethoxy-(1H)-quinone Preparation of lin-2-one p-toluate
[0030]
[0031] Add 5.0 g of 5,6-diethyl-2,3-dihydro-1H-indene-2-amine and 7 mL of dimethylmethylene into a four-necked flask equipped with magnetic stirring, thermometer, addition funnel and reflux condenser sulfone, 7.0 g of 5‐(2R)‐2‐oxiranyl‐8‐benzyloxy‐2(1H)‐quinolinone was added to the solution, the resulting suspension was heated to 110 °C, and Stirring at this temperature for 7h, TLC showed that the reaction was complete. Cool the resulting brown solution to 60°C, add 80mL of ethanol, keep at 60°C, then add 3.9g p-toluic acid to dissolve completely, cool the solution to about 40°C, add seed crystals, and place in an ice bath to cool. 10.0 g of the crude p-toluate salt was isolated by filtration and recrystallized from ethanol to give 9.4 g of 5‐[(R)‐2‐(5,6‐diethyl‐indan‐2‐ylamino)‐1‐hydroxy‐ The pure product of ethyl]‐8‐phenylm...
Embodiment 35
[0032] Example 3.5-[(R)-2-(5,6-diethyl-indan-2-ylamino)-1-hydroxy-ethyl]-8-phenylmethoxy-(1H)-quinone Preparation of lin‐2‐one p-nitrobenzoate
[0033]
[0034] Add 5.0 g of 5,6-diethyl-2,3-dihydro-1H-indene-2-amine and 7 mL of dimethylmethylene into a four-necked flask equipped with magnetic stirring, thermometer, addition funnel and reflux condenser sulfone, 7.0 g of 5‐(2R)‐2‐oxiranyl‐8‐benzyloxy‐2(1H)‐quinolinone was added to the solution, the resulting suspension was heated to 110 °C, and Stirring at this temperature for 7h, TLC showed that the reaction was complete. Cool the resulting brown solution to 60°C, add 80mL of ethanol, keep at 60°C, then add 4.8g of p-nitrobenzoic acid to dissolve completely, cool the solution to about 40°C, add seed crystals, and place in an ice bath to cool. 11.2 g of crude p-methoxybenzoate were isolated by filtration and recrystallized from ethanol to give 9.8 g of 5‐[(R)‐2‐(5,6‐diethyl‐indan‐2‐ylamino)‐1‐ Hydroxy-ethyl]-8-phenylmethox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com