Preparation method of indacaterol maleate
A technology of acid indene and ethyl acetate, applied in the field of chemical substances, can solve problems such as high cost and difficult operation, and achieve the effects of convenient post-processing, improved product quality and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
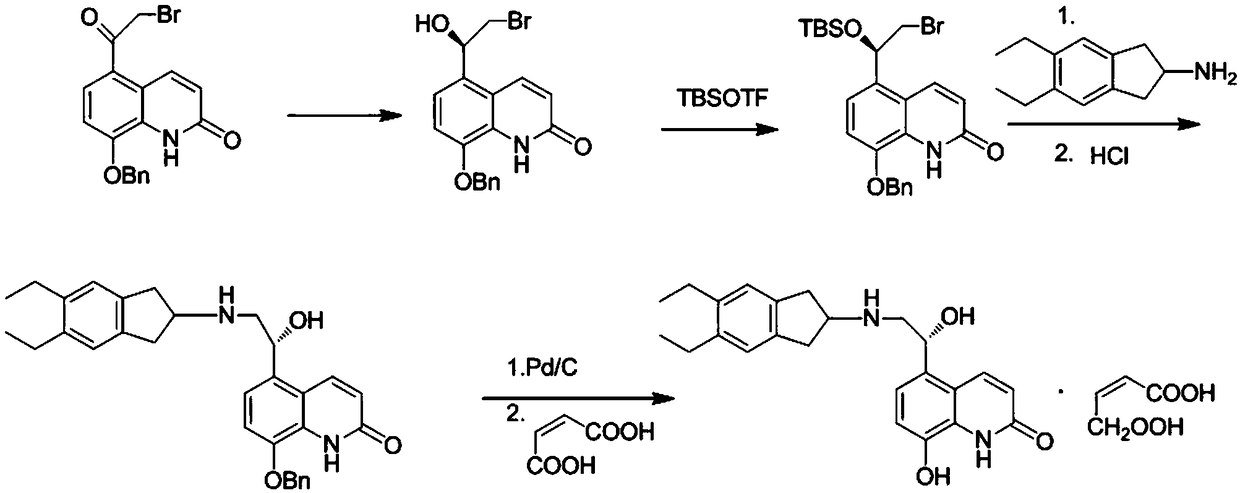
[0028] The present embodiment provides a kind of preparation method of indacaterol maleate, specifically comprises the following steps:
[0029] Step 1: Add 90g of 8-benzyloxy-5-(2-bromoacetyl)-2-hydroxyquinoline and 15ml (1.8M) of toluene solution of R-Me-CBS into THF900ml, nitrogen protection, ice salt Cool the bath down to -10°C, add 294ml (1M) of borane tetrahydrofuran solution dropwise, after 3h, continue the low temperature reaction for 1h; add 250ml methanol to quench, spin the reaction solution, add 300ml ethyl acetate and 300ml The phase was dried and spin-dried to obtain 72.5 g of intermediate 1, yield: 81%.
[0030] Step 2: Add 70.2g of intermediate 1 to 260ml of DMF, protect with nitrogen, cool down in an ice bath, slowly add 99.8g of TBSOTF and 40.3g of 2,6-lutidine, after the addition is complete, return to room temperature and react for 1h; add Quenched with 45ml of methanol, then added 500ml of ethyl acetate:cyclohexane=1:1 mixed solution and 500ml of saline; ...
Embodiment 2
[0034] The present embodiment provides a kind of preparation method of indacaterol maleate, specifically comprises the following steps:
[0035] Step 1: Add 90g of 8-benzyloxy-5-(2-bromoacetyl)-2-hydroxyquinoline and 15ml (1.8M) of toluene solution of R-Me-CBS into THF900ml, nitrogen protection, ice salt Cool the bath down to -10°C, add 294ml (1M) of borane tetrahydrofuran solution dropwise, after 3h, continue the low temperature reaction for 1h; add 250ml methanol to quench, spin the reaction solution, add 300ml ethyl acetate and 300ml The phase was dried and spin-dried to obtain 71 g of intermediate 1 with a yield of 79%.
[0036] Step 2: Add 70.2g of intermediate 1 to 260ml of DMF, protect with nitrogen, cool down in an ice bath, slowly add 99.8g of TBSOTF and 40.3g of 2,6-lutidine, after the addition is complete, return to room temperature and react for 1h; add Quenched with 45ml of methanol, then added 500ml of ethyl acetate:cyclohexane=1:1 mixed solution and 500ml of sa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com