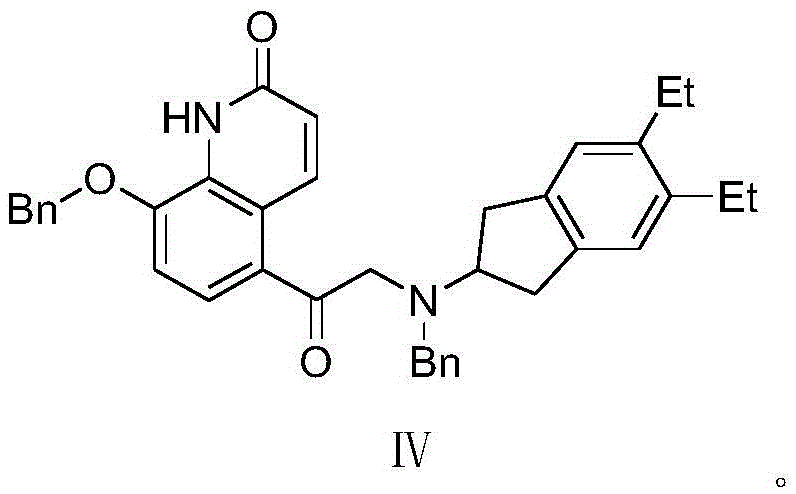
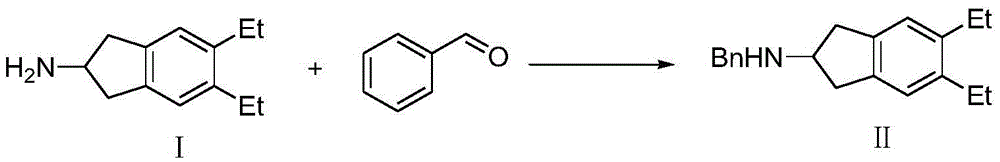
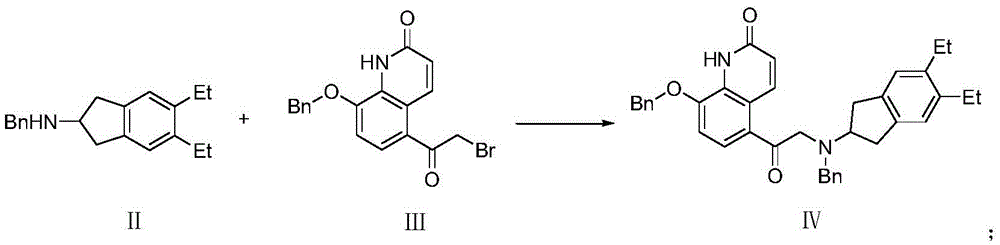
The synthetic method of indacaterol intermediate and indacaterol
A synthetic method and intermediate technology, applied in the field of drug synthesis, can solve the problems of high impurity content, high production cost, and low product yield, and achieve the effects of simple operation, low cost, and avoidance of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] 1.N-benzyl-5,6-diethyl-2,3-dihydro-1H-inden-2-amine (formula II compound) synthesis
[0047]Under nitrogen protection, add 6.00g 5,6-diethyl-2,3-dihydro-1H-indene-2-amine (compound of formula I), 60.00ml methanol and 4.50g benzaldehyde, oil The bath was warmed to 65°C for 4 hours. Then slowly lower the temperature to -25°C, add 4.80g of sodium borohydride in batches, keep the internal temperature below 25°C, after the addition is complete, slowly raise the temperature to room temperature, and react overnight. Most of the methanol was removed by rotary evaporation, 50.00ml of water was added dropwise to the residue, and then 50.00ml of ethyl acetate was added for separation. The aqueous phase was back-extracted three times with 20.00ml of ethyl acetate, and the organic phases were combined. Dry over anhydrous sodium sulfate, filter, and concentrate to remove most of the solvent. 50ml of HCl / MeOH (wt=32%) was slowly added dropwise to the obtained solid-liquid mixture. A...
Embodiment 2
[0059] 1.N-benzyl-5,6-diethyl-2,3-dihydro-1H-inden-2-amine (formula II compound) synthesis
[0060] Under nitrogen protection, add 6.00 g of 5,6-diethyl-2,3-dihydro-1H-inden-2-amine (compound of formula I ), 60.00ml ethanol and 2.00g benzaldehyde, 1.00g NaBH 3 CN, the temperature of the oil bath was raised to 80°C for 4 hours. Rotary evaporation to remove most of the ethanol, drop 50.00ml of water into the residue, then add 50.00ml of ethyl acetate to separate the liquid, back extract the aqueous phase with 20.00ml of ethyl acetate for 3 times, combine the organic phases, and wash with anhydrous sulfuric acid Dry over sodium, filter, and concentrate to remove most of the solvent. To the obtained solid-liquid mixture, 50.00 ml of HCl / MeOH (wt=32%) was slowly added dropwise. After dropping, the mixture was stirred for 1 hour and then filtered to obtain a white solid (dry weight 5.50 g, yield 91%, purity 99%).
[0061] 1 H NMR (400MHz, CD 3 OD): δ7.61-7.45(m, 5H), 7.10(s, 2...
Embodiment 3
[0072] 1.N-benzyl-5,6-diethyl-2,3-dihydro-1H-inden-2-amine (formula II compound) synthesis
[0073] Under nitrogen protection, add 6.00 g of 5,6-diethyl-2,3-dihydro-1H-inden-2-amine (compound of formula I) to the reaction flask at -25°C (cooling with dry ice-acetone bath) , 60.00ml THF and 6.70g benzaldehyde, 34.00g NaBH (OAc) 3 , the temperature of the oil bath was raised to 80° C. for 4 hours. Most of the THF was removed by rotary evaporation, 50.00 ml of water was added dropwise to the residue, and then 50.00 ml of ethyl acetate was added for separation. The aqueous phase was back-extracted 3 times with 20.00ml of ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, concentrated to remove most of the solvent, and slowly added dropwise 50.00ml of HCl / MeOH (wt =32%), dropwise, and filtered after stirring for 1 hour to obtain a white solid (dry weight 5.50 g, yield 91%, purity 99%).
[0074] 1 H NMR (400MHz, CD 3 OD): δ7.61-7.45(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com