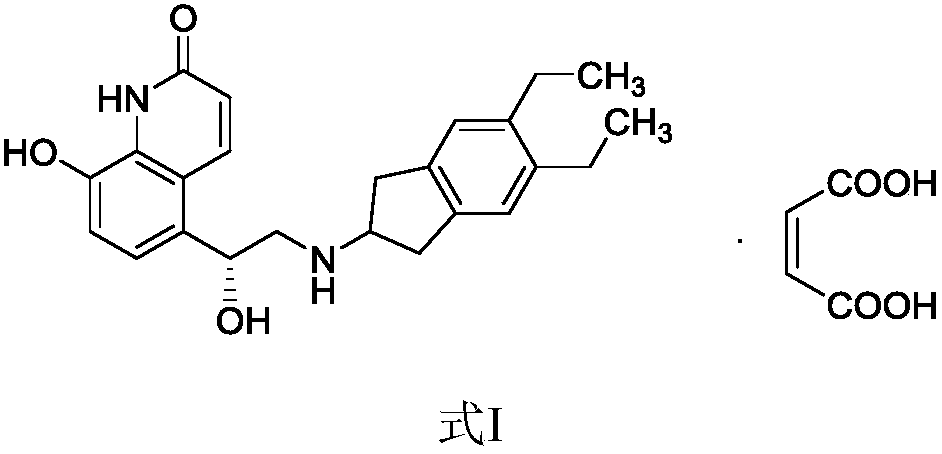
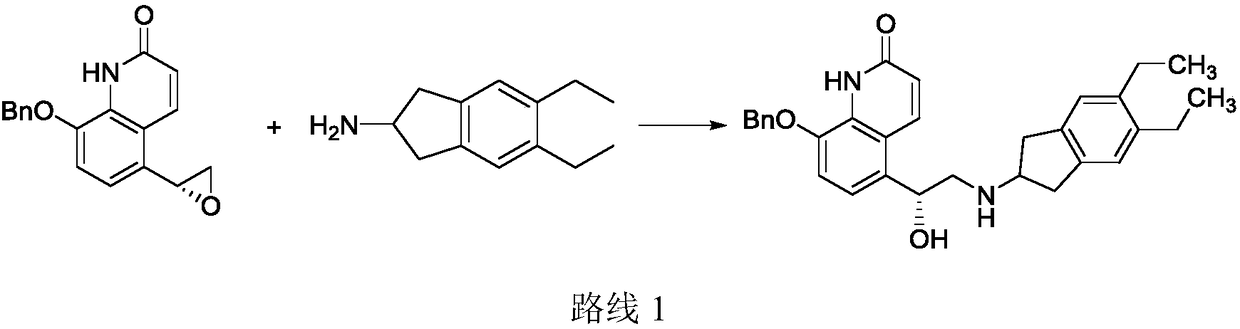
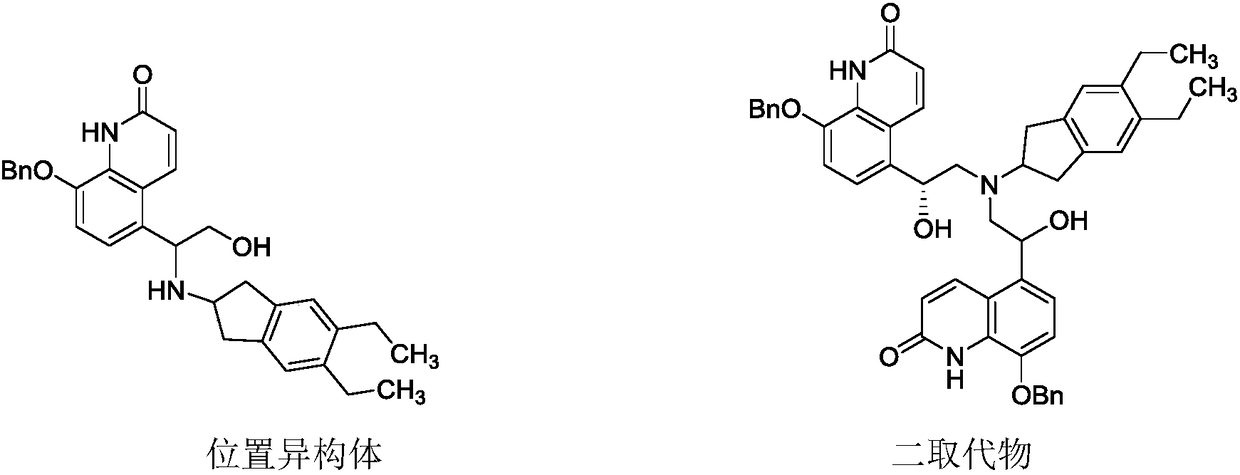
Preparation method for indacaterol maleate
A technology of acid addition salts and compounds, applied in the field of drug synthesis, can solve problems such as multiple secondary substitutions and positional isomer impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The preparation of embodiment 1 compound IIA
[0064]
[0065] Prepare NaOH aqueous solution ①: Add 32g of sodium hydroxide to 1000ml of purified water, stir to dissolve, and set aside for later use. Prepare NaOH aqueous solution ②: add 16g of sodium hydroxide to 1000ml of purified water, stir to dissolve, and set aside for later use. Add 90 g of compound IIA hydrochloride, 50 ml of methanol and NaOH aqueous solution ① into the reaction flask, stir for 30 minutes, extract with 1600 ml of dichloromethane in two equal parts, combine the organic phases; wash once with NaOH aqueous solution ②, and use 2000 ml of purified water to Wash twice; the organic phase was stirred and dried with anhydrous sodium sulfate for 1 h; the desiccant was filtered off, and the filtrate was evaporated to dryness.
Embodiment 2
[0066] The preparation of embodiment 2 indacaterol intermediate II hydrobromide
[0067]
[0068] Add the residue compound IIA, 125g of compound IIB-1 and 1300ml of n-butanol solution obtained in Example 1 to dryness into a 5L reaction flask, stir evenly, heat to 100-110°C, and start TLC detection for 3 hours until compound IIA reacts Turn off the heat when complete. Evaporate to dryness at 50-60°C under reduced pressure, transfer to a 3L reaction flask, add 1000ml ethyl acetate, heat to 70-78°C, reflux and beat for 1.5h; filter with suction, dry the filter cake at 50°C under reduced pressure for 5h to obtain 174.4g The hydrobromide of formula II is a dark brown solid powder with a yield of 93% and a liquid phase purity of 97.82%. Its element analysis data is: C 31 h 33 BrN 2 o 3 Found (theoretical) %: C 66.25 (66.31), H 5.93 (5.92), O 8.56 (8.55), N 4.99 (4.99), Br 14.22 (14.23).
Embodiment 3
[0069] The preparation of embodiment 3 indacaterol intermediate III-2
[0070]
[0071] Add indacaterol intermediate II-1 and tetrahydrofuran into the reaction flask, stir evenly, under nitrogen protection, and ice-water bath to lower the reaction temperature to 0-3°C, add (R)-2-methyl-CBS-oxazole boron Alkanes, and then dropwise added tetrahydrofuran borane solution, about 30min dropwise. Continue to keep the reaction internal temperature at 0-3°C for 6-8 hours until the reaction is complete as monitored by TLC. The reaction was quenched by adding methanol solution dropwise, the reaction liquid was evaporated under pressure to remove the solvent, slurried with 1M dilute hydrochloric acid for 3 hours, and filtered to obtain an off-white solid. Air-dried at 60°C for 6 hours to obtain 7.5 g of the hydrobromide salt of formula III (formula III-1) as an off-white solid with a liquid phase purity of 97.86%.
[0072] Add the hydrobromide salt of indacaterol intermediate III (fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com