Indacaterol maleate intermediate, and preparation method and application thereof
A fumaric acid, solid technology, applied in the direction of organic chemistry, bulk chemical production, etc., can solve the problems of poor purification effect of enantiomer impurities, no purification effect, limited purification effect, etc., and achieves good removal effect, Good thermodynamic stability, good for storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: (R)-8-(benzyloxy)-5-[2-[(5,6-diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1 -Hydroxyethyl]quinoline-2(1H)-one (the compound of formula IV)
[0062] Add about 1.21Kg of 5,6-diethyl-2,3-dihydro-1H-inden-2-ylamine (compound of formula II), 10.62Kg of tert-butanol, and 3.00Kg of dimethyl sulfoxide into the reactor. Add (R)-5-(2-oxiranyl)-8-benzyloxy-2(1H)-quinolinone (compound of formula Ⅲ) 1.36Kg under stirring, heat and reflux for 18 hours and then cool to 25 At °C, 15.25Kg of ethyl acetate and 27.24Kg of purified water were added, and after stirring, it was allowed to stand for liquid separation. The organic phase is washed 6 times with 15.93Kg of saturated brine each time, and concentrated under reduced pressure until no obvious fraction flows out, stop the concentration to obtain (R)-8-(benzyloxy)-5-[2-[(5,6 -Diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1-hydroxyethyl]quinoline-2(1H)-one (compound of formula IV), HPLC chemical purity: 63.6% (Among them, impurity for...
Embodiment 2
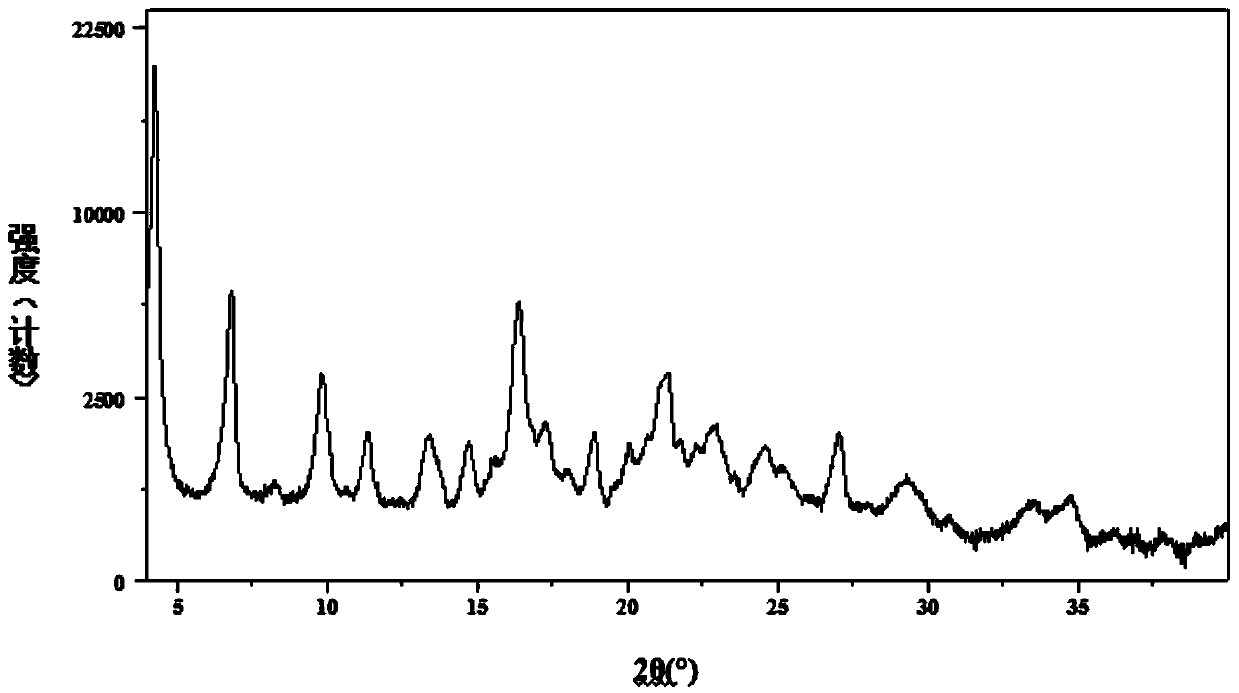
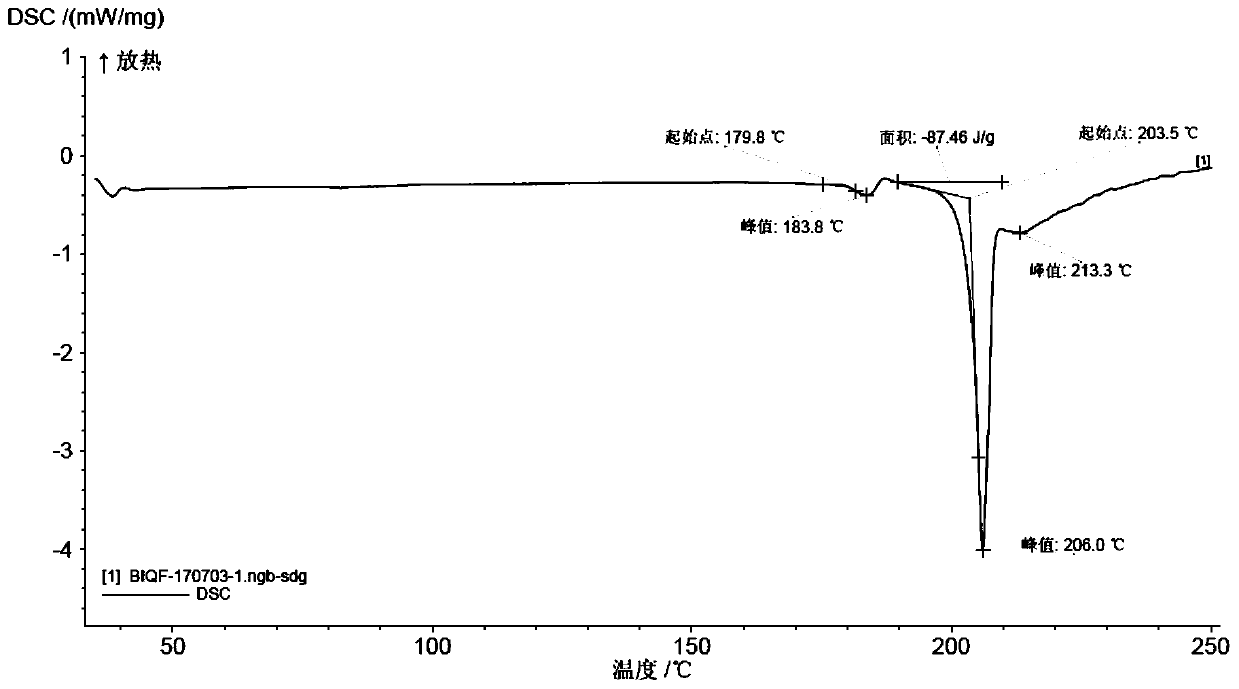
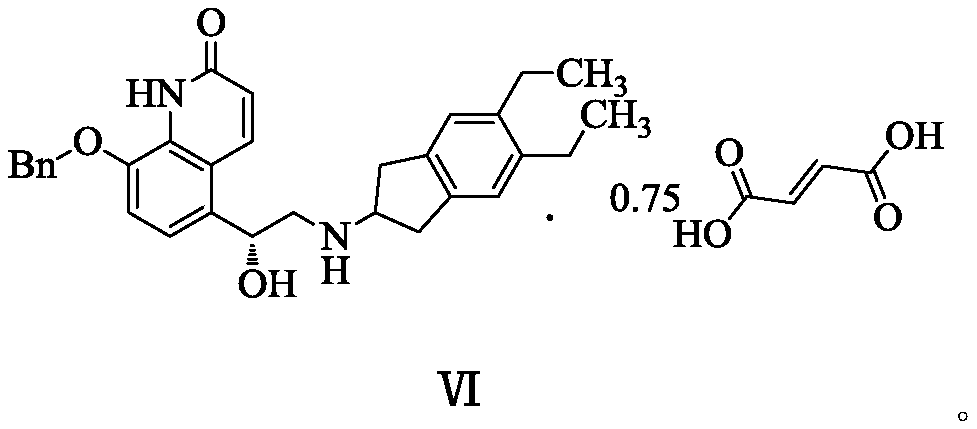
[0063] Example 2: (R)-8-(benzyloxy)-5-[2-[(5,6-diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1 -Hydroxyethyl]quinoline-2(1H)-one·0.75 fumarate (compound of formula VI) and preparation of its crystal form A
[0064] Add 160 g of anhydrous ethanol to the reaction flask, add 23 g (content 63.6%, 0.03 mol) of the compound of formula IV obtained above, stir and raise temperature and reflux to dissolve, then add 7.0 g (0.06 mol) of fumaric acid. After the addition, continue to reflux and stir to crystallize for about 2 hours. Cool down to 25°C. Filter and wash the filter cake with an appropriate amount of absolute ethanol. The obtained filter cake was added to the reaction flask, 120 g of absolute ethanol was added, and the temperature was raised to reflux for about 2 hours. Cool down to about 25°C. Filter and wash the filter cake with an appropriate amount of absolute ethanol. Then, it was put into a vacuum drying oven and dried under reduced pressure at 50-60°C to obtain 16.2g of the...
Embodiment 3
[0073] Example 3: (R)-8-(benzyloxy)-5-[2-[(5,6-diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1 -Hydroxyethyl]quinoline-2(1H)-one·0.75 fumarate and its crystal form A (formula VI compound) preparation
[0074] Add 300 g of absolute ethanol to the reaction flask, add 23 g (content 63.6%, 0.03 mol) of the compound of formula IV obtained above, stir and raise the temperature to 40-50° C. to dissolve, then add 4.2 g (0.036 mol) of fumaric acid. After the addition, continue to stir for about 2 hours. Cool down to 0~5℃. Filter and wash the filter cake with an appropriate amount of absolute ethanol. Then it is put into a vacuum drying oven and dried under reduced pressure at 30-40°C to obtain the crystal form A of the compound of formula VI. HPLC chemical purity is 98.9%. (Among them, the impurity formula II compound is 0.1%, the impurity formula IV-a compound is 0.1%, the impurity formula IV-b compound is 0.3%, and the maximum single impurity is 0.3%), and the HPLC optical purity is 99.8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com