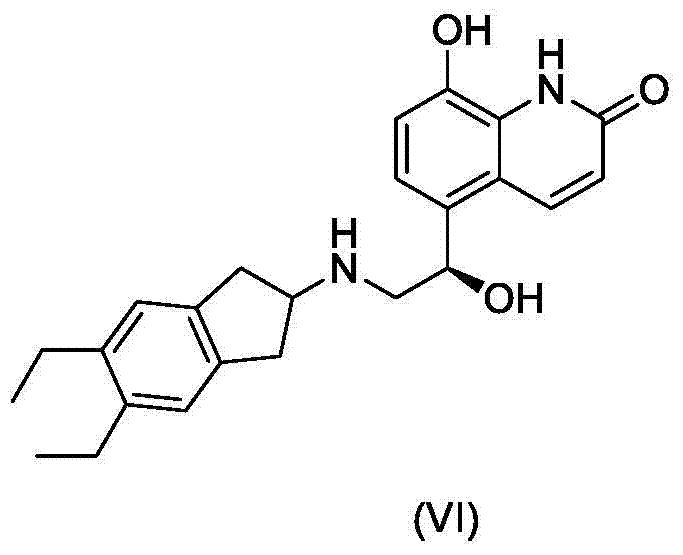
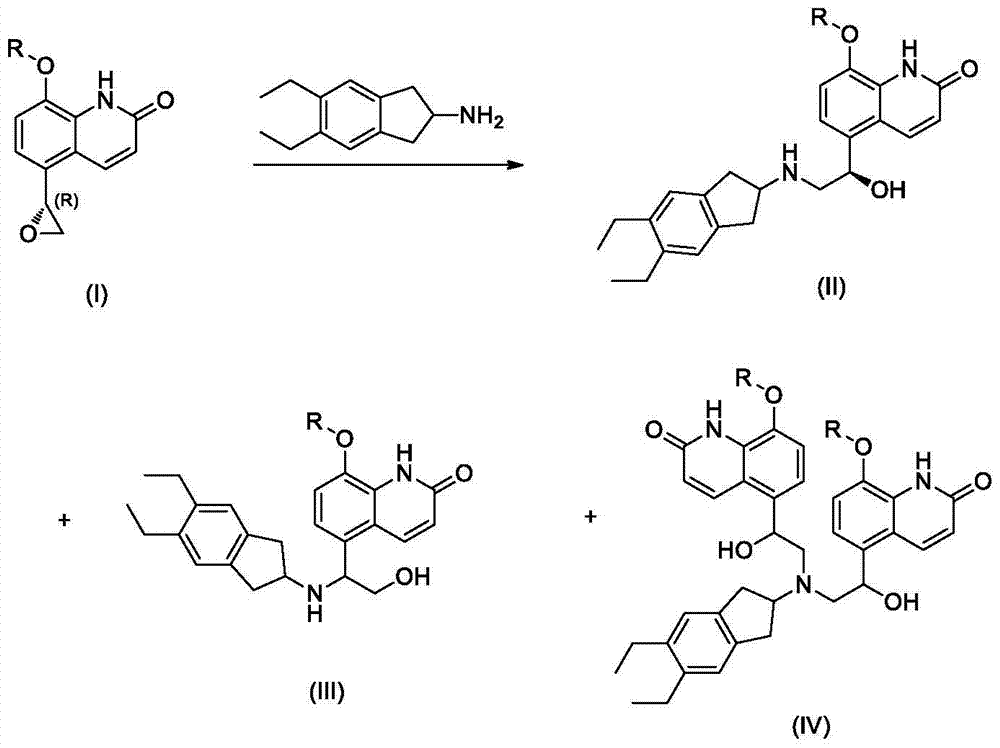
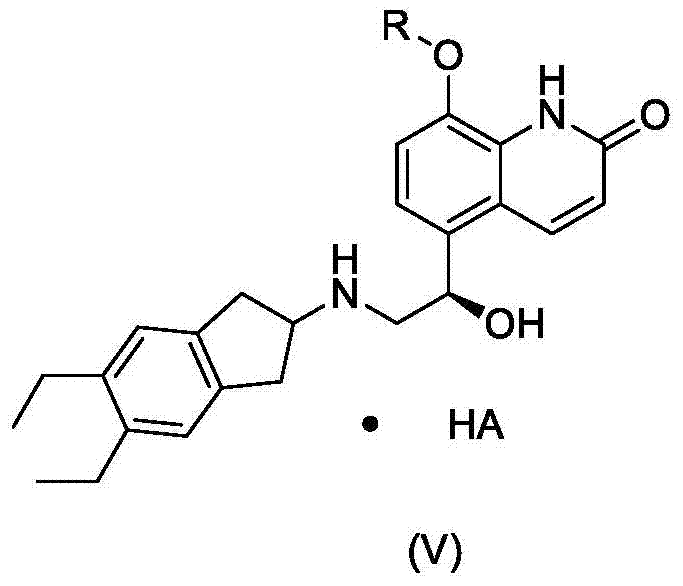
Indacaterol intermediate salt and preparation method thereof
An intermediate, selected technology, applied in the field of salt of indacaterol intermediate and its preparation, can solve the problems of unsatisfactory yield and purity, and achieve the effect of low cost, easy realization and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (R)-5-[2-[(5,6-diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1-hydroxyethyl]-8-(benzyloxy )-2 (1H) - preparation of quinolinone malate:
[0034] 8-Benzyloxy-5-(R)-oxiranyl-(1H)-quinolin-2-one (1.2 g) and 2-amino-(5,6-diethyl)-indan (1.1 g) was added to diethylene glycol dimethyl ether, heated to 110 ° C, and reacted at this temperature for 48 hours, the resulting suspension was cooled to 70 ° C, ethanol (70 ml) was added, followed by malic acid (0.6 g), the solution was cooled to 20-30° C., seed crystals were added, the resulting suspension was cooled to 0-5° C., the solid was separated by filtration, and vacuum-dried. The yield was 64%, and the HPLC purity was 98.4%.
Embodiment 2
[0036] (R)-5-[2-[(5,6-diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1-hydroxyethyl]-8-(benzyloxy )-2 (1H) - preparation of quinolinone oxalate:
[0037] 8-Benzyloxy-5-(R)-oxiranyl-(1H)-quinolin-2-one (1.2 g) and 2-amino-(5,6-diethyl)-indan (1.1 g) was added to n-butanol, heated to 120°C, and reacted at this temperature for 48 hours, the resulting suspension was cooled to 70°C, oxalic acid (0.5 g) was added, and the solution was cooled to 20-30°C , adding seed crystals, cooling the resulting suspension to 0-5° C., separating the solid by filtration, and drying in vacuo. The yield is 65%, and the HPLC purity is 98.6%.
Embodiment 3
[0039] (R)-5-[2-[(5,6-diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1-hydroxyethyl]-8-(trityl Preparation of (oxy)-2(1H)-quinolinone malonate:
[0040] 8-trityloxy-5-(R)-oxiranyl-(1H)-quinolin-2-one (1.2 g) and 2-amino-(5,6-diethyl) - Indane (1.1 g) was added to diethylene glycol dimethyl ether, heated to 110 ° C, and reacted at this temperature for 48 hours, the resulting suspension was cooled to 70 ° C, and malonic acid (0.7 g) was added , the solution was cooled to 20-30° C., seed crystals were added, the resulting suspension was cooled to 0-5° C., the solid was separated by filtration, and vacuum-dried. The yield was 68%, and the HPLC purity was 99.0%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com