Process for preparing fluticasone diproprionate superfine particles and product thereof
A technology of fluticasone propionate and fine particles, which is applied in the production of products, bulk chemicals, steroids, etc., can solve the problems of low bioavailability of human body, long development cycle, and difficult research and development, so as to facilitate human body absorption and product High quality, enhanced solubility and efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
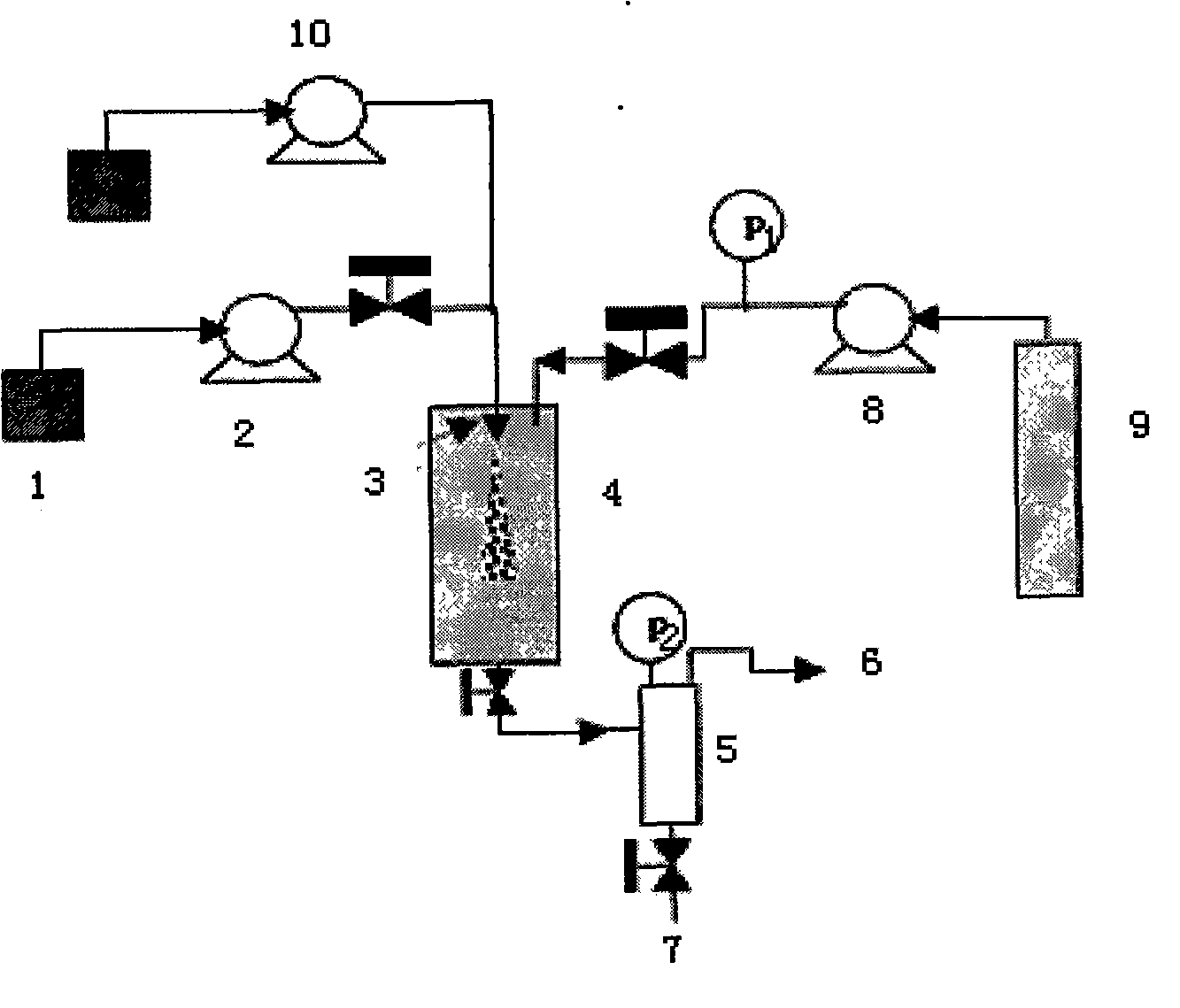
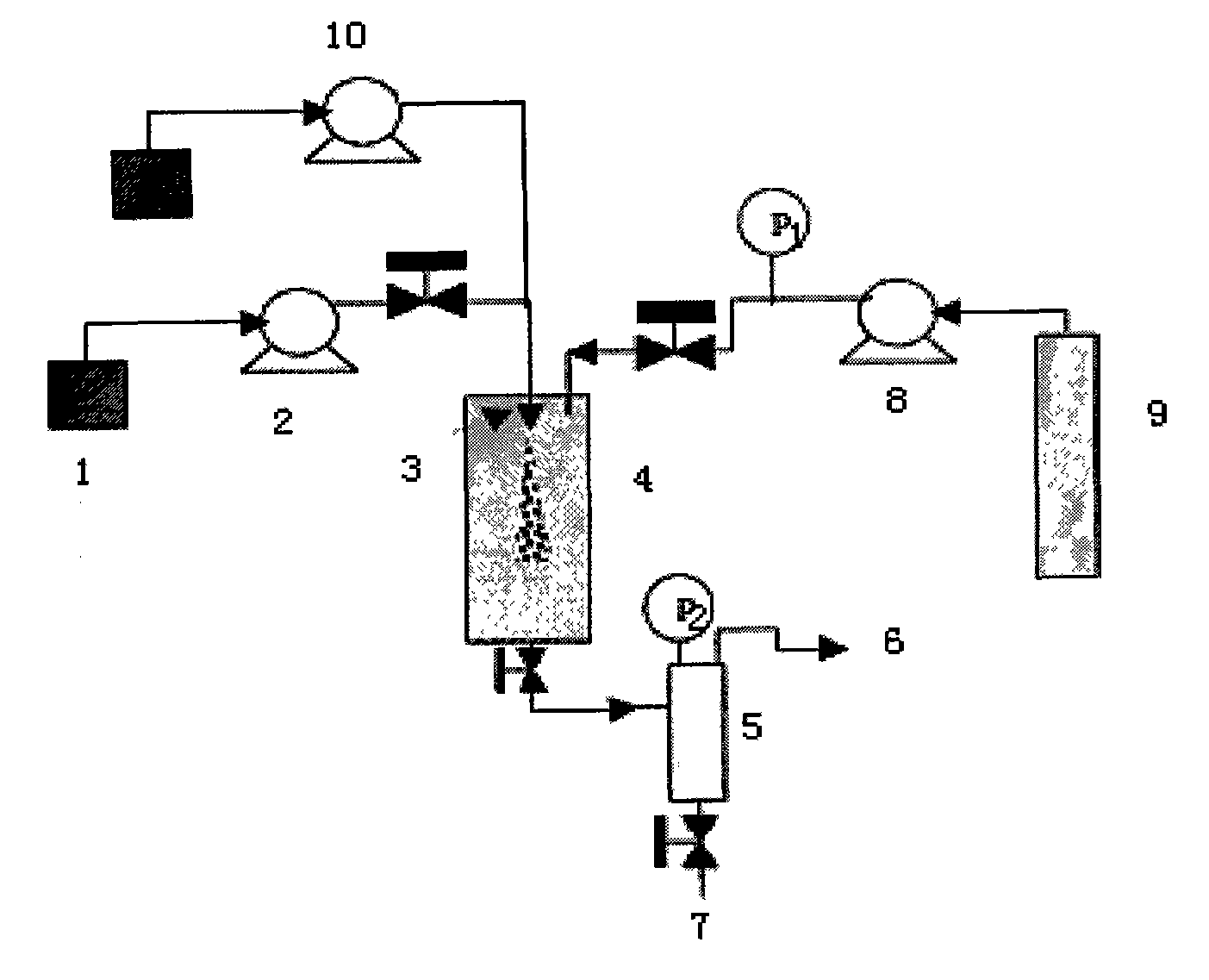
[0027] Embodiment: a kind of preparation technology of fluticasone propionate fine particle is characterized in that it comprises the following steps:
[0028] (1) configure fluticasone propionate solution: according to the ratio of organic solvent: fluticasone propionate 200:3, fluticasone propionate is fully dissolved in the organic solvent, so that the concentration of fluticasone propionate is: 1.5%; the dissolution temperature is controlled at 50 ℃;
[0029] (2) the fluticasone propionate solution 1 prepared in the step (1) is connected to the solution pump 2, and the working pressure is controlled to be 15MPa;
[0030] (3) Carbon dioxide feed: the CO in the cylinder 2 9. Enter the supercritical fluid anti-solvent equipment system through the booster pump 8, enter the crystallization kettle 4, control the flow rate at 10ml / min, control the starting temperature at 50°C, and the pressure at 15MPa;
[0031] (4) The fluticasone propionate solution configured in the above-me...
PUM
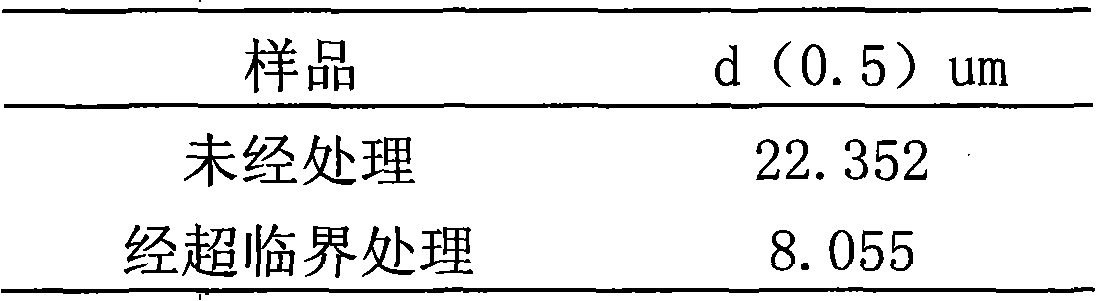
| Property | Measurement | Unit |
|---|---|---|
| Effective particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com