Tiotropium bromide anhydride and preparation method thereof
A technology of tiotropium bromide and anhydrate, applied in the field of tiotropium bromide anhydrate and its preparation, can solve the problem of affecting the quality and yield of intermediate II, affecting the quality of final product tiotropium bromide anhydrate, High-quality alcohol esters and other issues, to achieve safe production and environmental protection benefits, good quality, and reduce impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
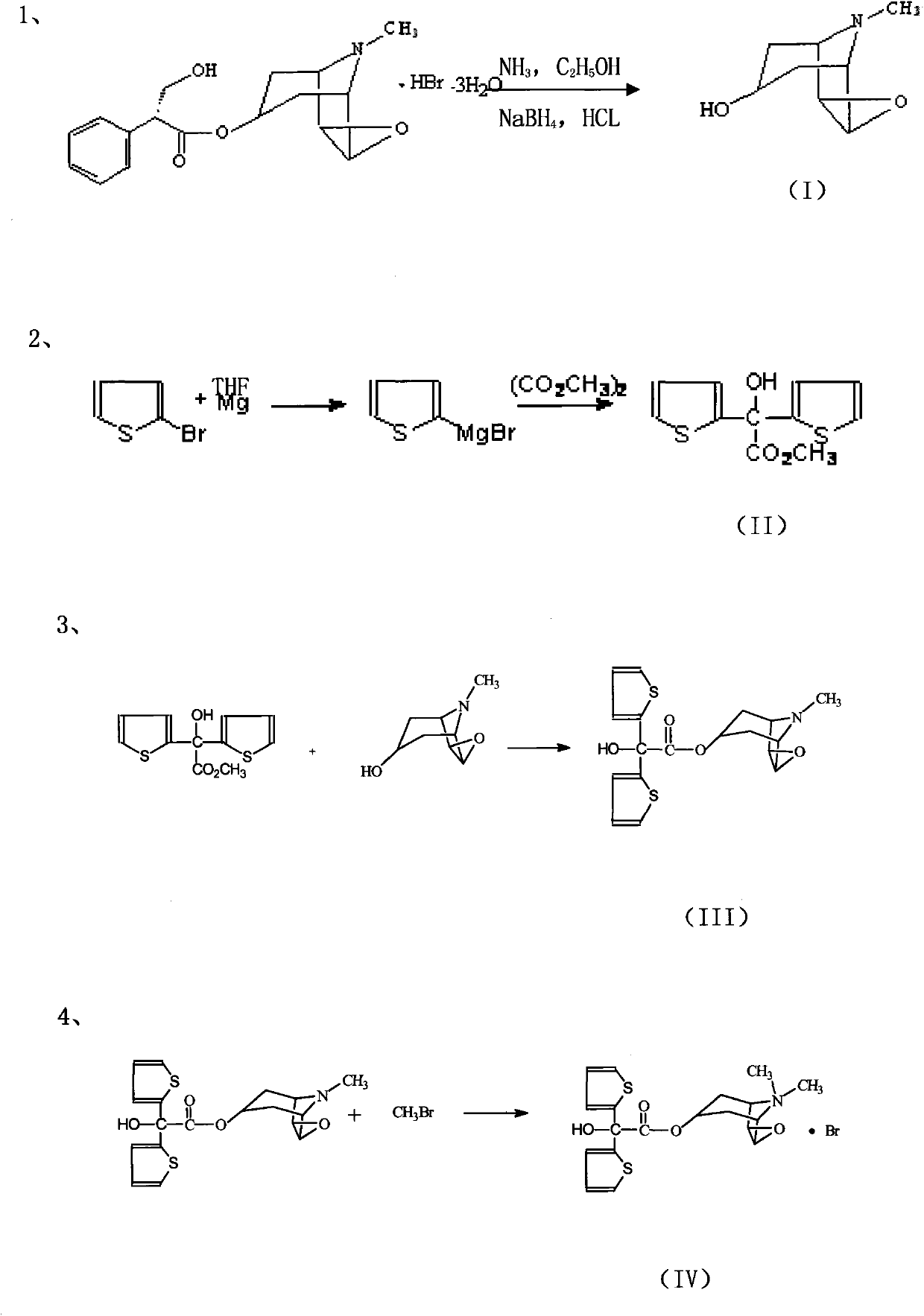
[0029] 1, the preparation of scopolamine (intermediate I)
[0030] Under controlled anhydrous and anaerobic conditions, use a special bottom valve and a multifunctional reactor as a reactor, and use a special filter as a refining device, take 5.7g (0.013mol) of scopolamine hydrobromide, add 60% dehydrated alcohol ~80ml, alkalized to pH8~9 with ammonia gas. Add about 5.1 g (0.1353 mol) of sodium borohydride in portions, stir the reaction at 10-20°C, follow the reaction with TLC, and the reaction time is about 10-12 hours. Hydrogen chloride was acidified to pH 1~2, diluted with 70~130ml of anhydrous ether, and filtered. Add 50-70ml of dichloromethane to the filter cake, and alkalinize it with ammonia gas to pH 7-8. Suction filter, wash with 30-50ml dichloromethane in 3 times. The filtrates were combined and evaporated to dryness under reduced pressure below 25°C to obtain Intermediate I as a white solid with a yield of about 83%.
[0031] 2, the preparation of two (2-thienyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com