Method for preparing tiotropium bromide
A technology of tiotropium bromide and thiophene, which is applied in the field of preparation of tiotropium bromide, can solve the problems of long time, low yield, and many side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
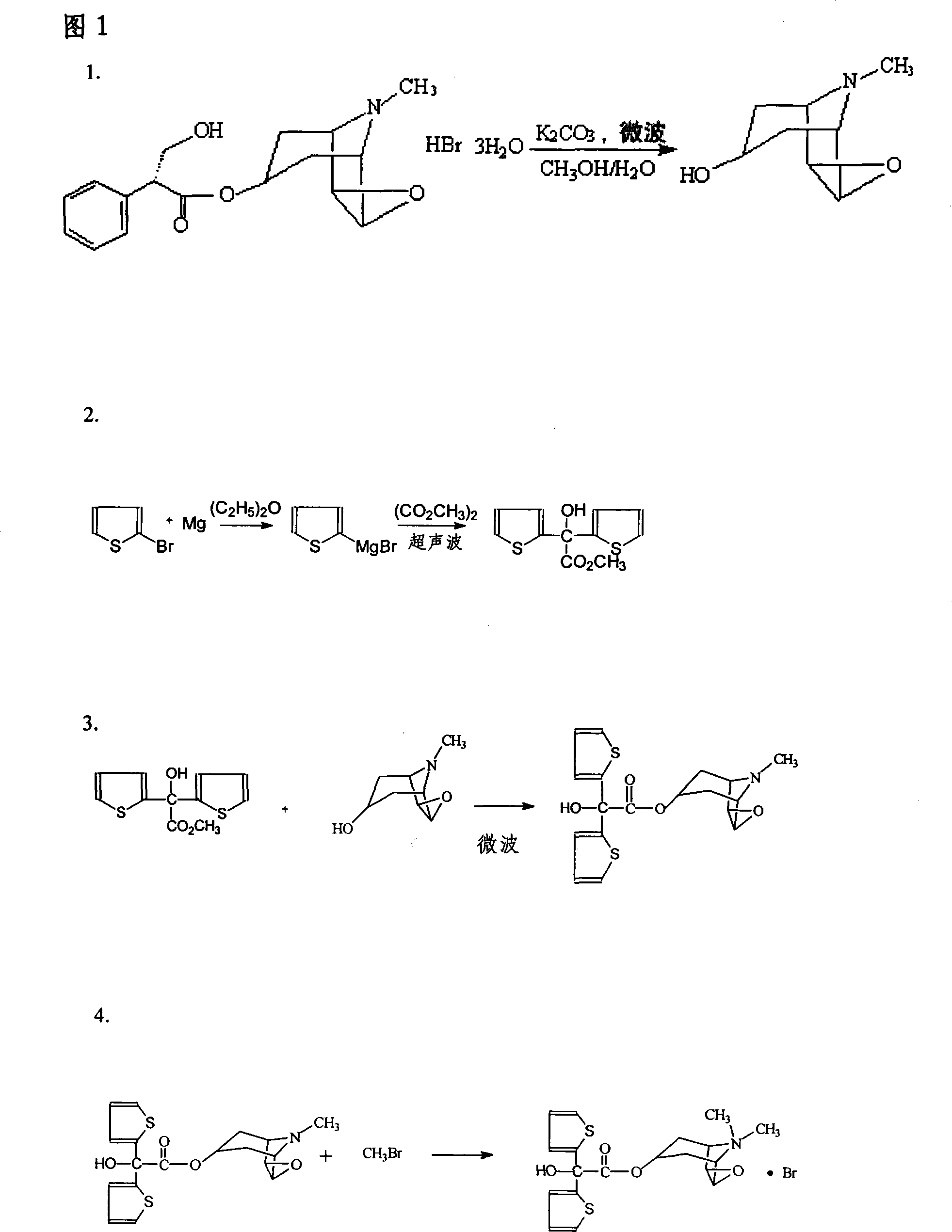
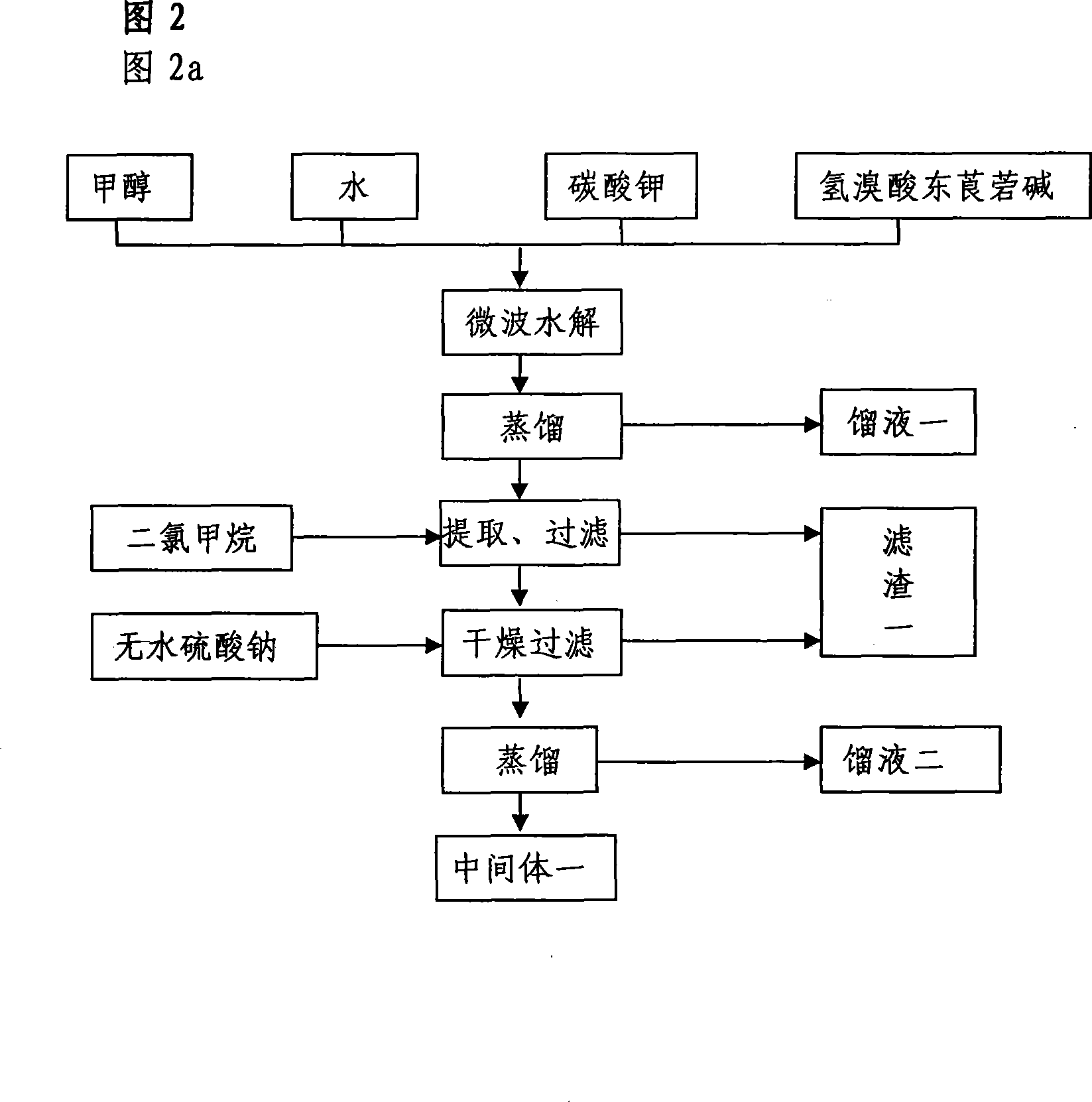
[0006] Preparation of Scopolamine (Intermediate 1)
[0007] Take scopolamine hydrobromide, methanol, and potassium carbonate, put them in a closed microwave normal pressure reactor with a wavelength of 0.1-100 cm, react quickly at room temperature, evaporate to dryness under reduced pressure, add dichloromethane, filter, filter cake with di Wash with methyl chloride three times, combine the filtrates, dry over anhydrous sodium sulfate, and evaporate to dryness under reduced pressure to obtain scopolamine oil (intermediate 1) with a yield of 99.3%.
Embodiment 2
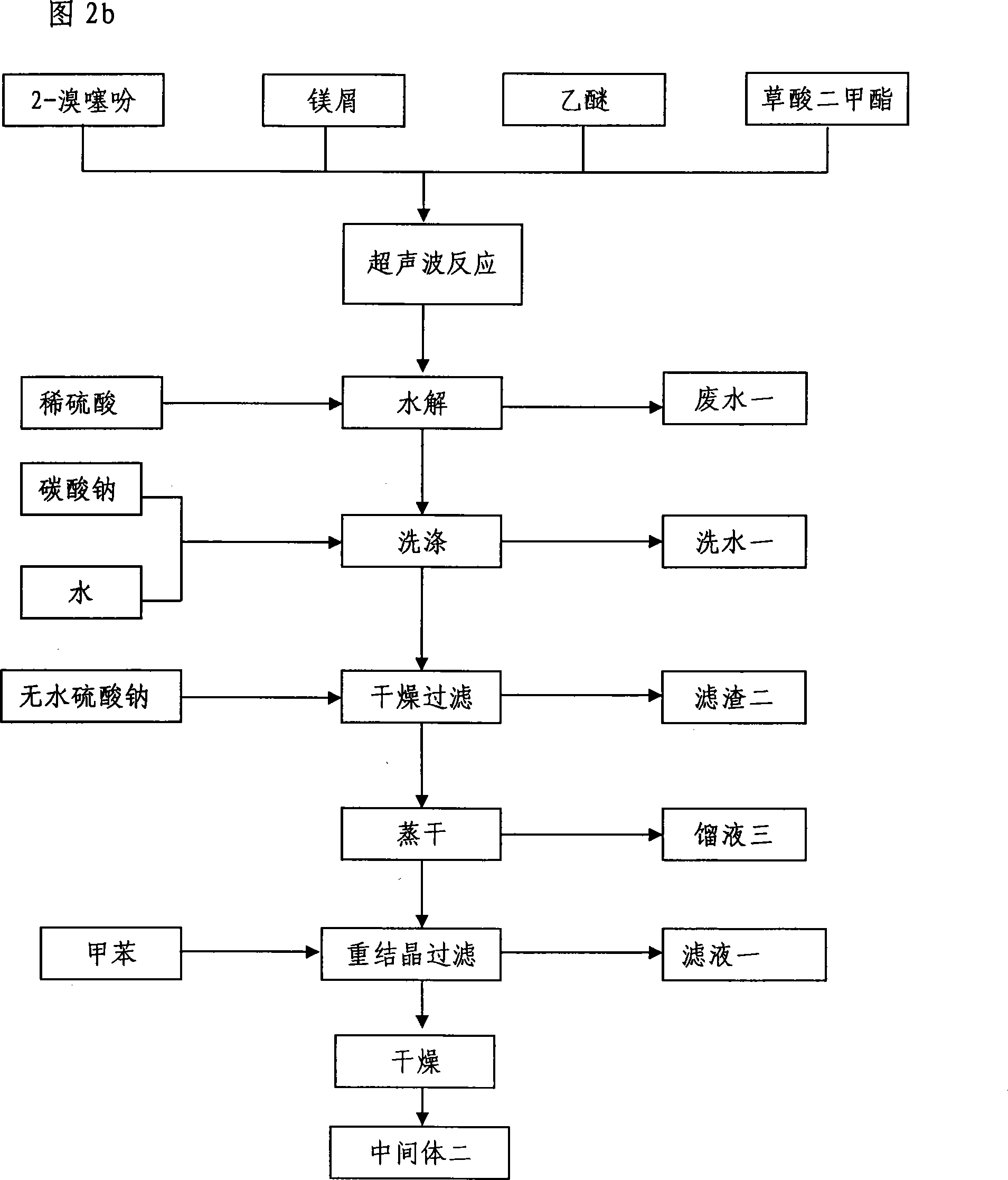
[0009] Preparation of bis(2-thienyl)glycolic acid methyl ester (intermediate 2)
[0010] Take bromothiophene, magnesium chips, dimethyl oxalate, and ether, and place them in an ultrasonic reactor with a frequency of 20-300KHZ. After rapid reaction at room temperature, add dilute sulfuric acid dropwise to the reaction solution, separate the ether layer, Wash with sodium aqueous solution, wash with water, dry over anhydrous sodium sulfate, evaporate to dryness, and recrystallize with toluene to obtain bis(2-thienyl)glycolic acid methyl ester crystals (intermediate 2) with a yield of 96.7%.
Embodiment 3
[0012] Preparation of Scopolamine Bis(2-thienyl)glycolate (Intermediate 3)
[0013] Mix Intermediate 2 and Intermediate 1 with an equimolar molecular ratio, add sodium and place in a closed microwave normal pressure reactor with a wavelength of 0.1 to 100 cm. After rapid reaction at room temperature, add toluene to the reaction solution and continue the reaction. Dilute with ethyl acetate, wash the organic layer twice with water, dry over anhydrous sodium sulfate, filter, evaporate to dryness, and recrystallize with acetonitrile to obtain crystals of scopolamine bis(2-thienyl)glycolate (intermediate 3) , the yield was 98.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com