Preparation method of Fe-doped oxyhalogen bismuth nanometer material
A technology of nanomaterials and bismuth oxyhalides, which is applied in nanotechnology, nanotechnology, chemical instruments and methods, etc., can solve the problems of long reaction time and unfavorable popularization and application, and achieve short reaction time, reduce production cost and save energy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A preparation method of Fe-doped bismuth oxyhalide nanomaterial (Fe-doped BiOCl) includes the following steps:
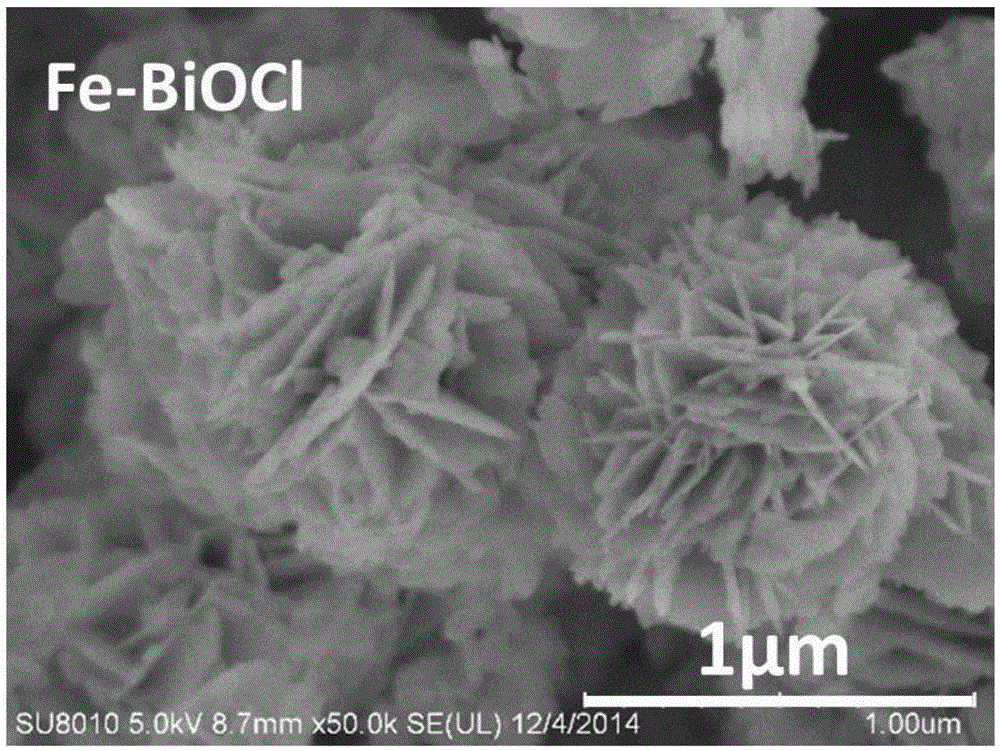
[0029] Dissolve 2mmol of bismuth nitrate pentahydrate in 50mL of ethylene glycol and mix well to obtain a mixed ethylene glycol solution, then dissolve 2mmol of potassium chloride and 0.5mmol of ferric nitrate nonahydrate in 40mL of deionized water and mix well to obtain a mixed aqueous solution; Add the prepared mixed aqueous solution dropwise to the mixed ethylene glycol solution at room temperature (25°C), place the resulting mixed solution in a 40KHz sonicator for ultrasonic dispersion (reaction) for 20 minutes, and centrifuge the resulting precipitate to remove residual solutes And solvent, and finally dried at 60 ℃ for 12h, cooling to obtain the final product.
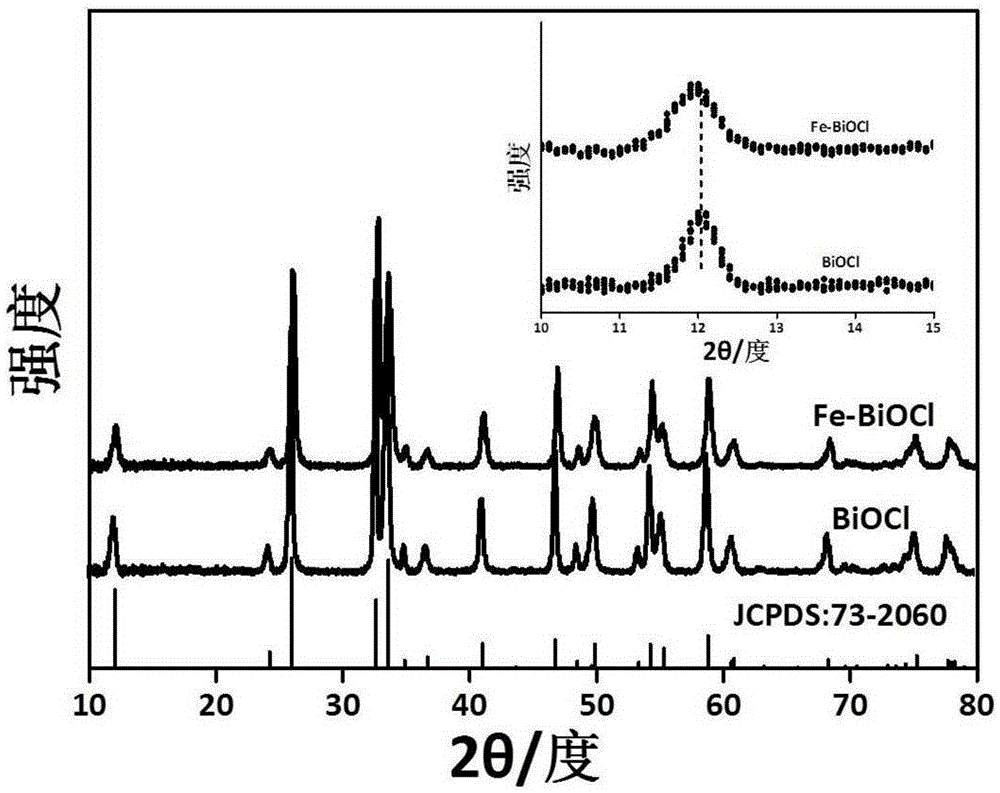
[0030] figure 1 This is the XRD pattern of the product obtained in the present invention. It can be seen from the pattern that the main peak is consistent with the standard pattern JCPDS: 73-2060, an...
Embodiment 2
[0033] A preparation method of Fe-doped bismuth oxyhalide nanomaterial (Fe-doped BiOBr) includes the following steps:
[0034] Dissolve 2mmol of bismuth nitrate pentahydrate in 40mL of ethylene glycol and mix well to obtain a mixed ethylene glycol solution, then dissolve 2mmol of potassium bromide and 1mmol of ferric nitrate nonahydrate in 50mL of deionized water and mix well to obtain a mixed aqueous solution; Add the prepared mixed aqueous solution dropwise to the mixed ethylene glycol solution at room temperature (25°C), place the resulting mixed solution in a 40KHz sonicator for ultrasonic dispersion (reaction) for 20 minutes, and centrifuge the resulting precipitate to remove residual solute and The solvent is finally dried at 60°C for 12 hours and cooled to obtain the final product.
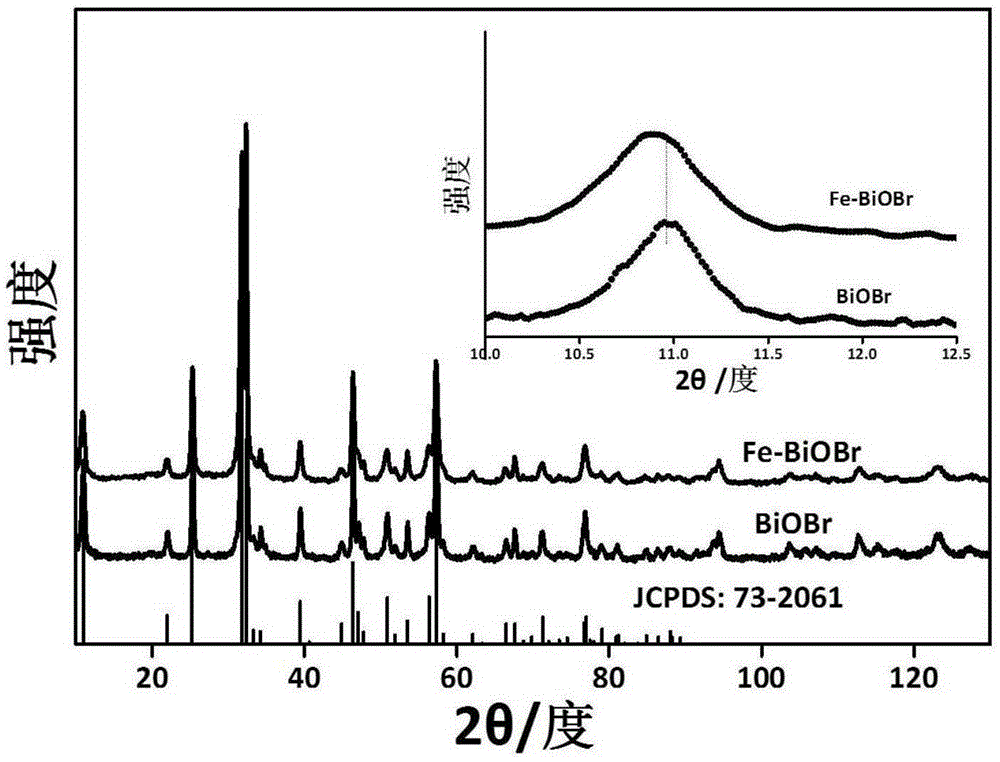
[0035] image 3 This is the XRD pattern of the product obtained in the present invention. It can be seen from the pattern that the main peak is consistent with the standard pattern JCPDS: 73-206...
Embodiment 3
[0039] A preparation method of Fe-doped bismuth oxyhalide nanomaterial (Fe-doped BiOCl) includes the following steps:
[0040] Dissolve 1mmol of bismuth nitrate pentahydrate in 30mL of ethylene glycol and mix well to obtain a mixed ethylene glycol solution, then dissolve 1mmol of potassium chloride and 1mmol of ferric nitrate nonahydrate in 100mL of deionized water and mix well to obtain a mixed aqueous solution; Add the prepared mixed aqueous solution dropwise to the mixed glycol solution at room temperature (20°C), place the resulting mixed solution in a 40KHz sonicator for ultrasonic dispersion (reaction) for 20 minutes, and centrifuge the resulting precipitate to remove residual solute and The solvent is finally dried at 60° C. for 12 hours and cooled to obtain the Fe-doped bismuth oxyhalide nanomaterial (Fe-doped BiOCl).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com